Chapter 2 Practice Problems
Answers for these practice problems are on the next page.
A good approach is to answer all of the questions on a piece of paper and then check your answers. This avoids accidentally seeing the answer(s) for questions you have not done yet.
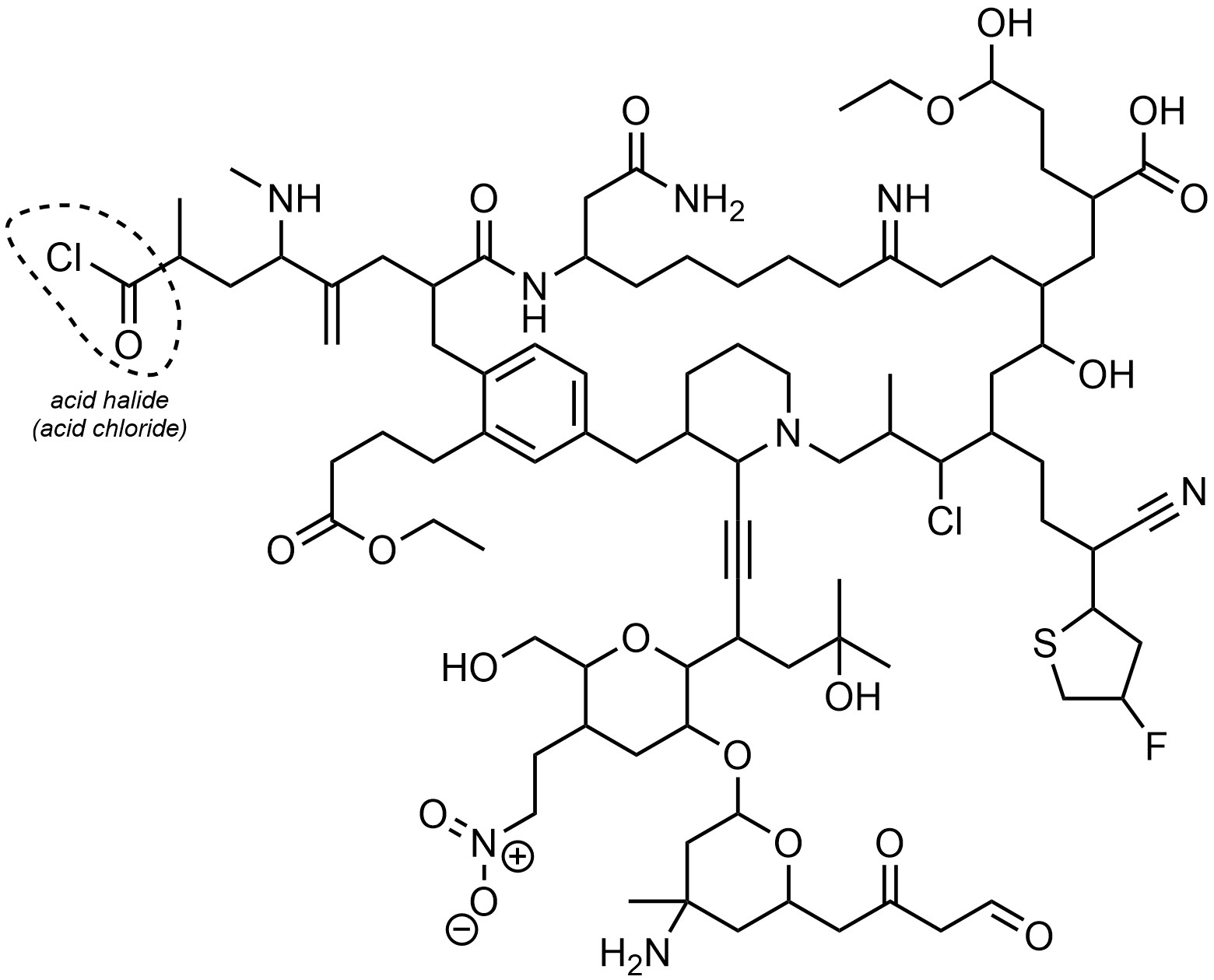
Q2.1: The following hypothetical molecule contains an impressive 25 functional groups. One of them has been circled and named for you. Circle and name the remaining functional groups in the same fashion. If possible, describe the subdivision (e.g. primary/secondary/tertiary, etc.). Functional groups may appear more than once. You may ignore alkanes.
Note: Because this molecule is very crowded you may wish to number the groups and name them in a list beside the molecule.
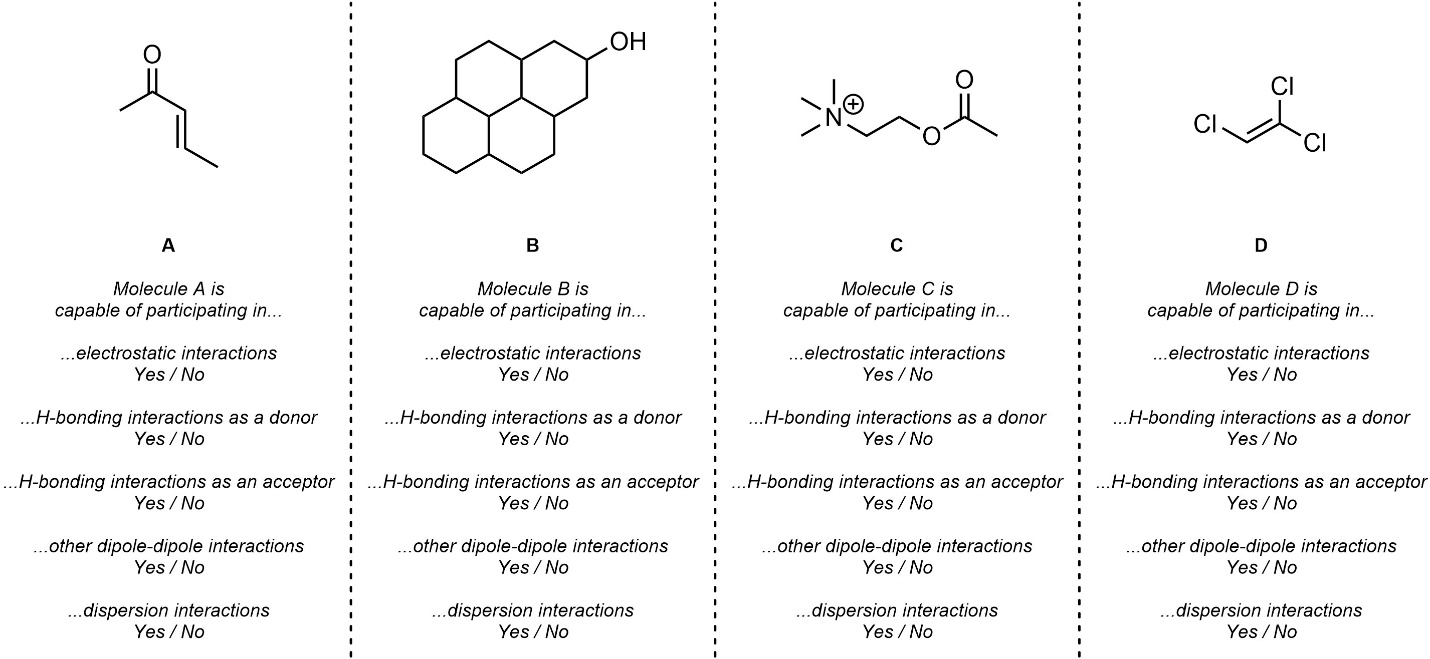
Q2.2: Which intermolecular force(s) is/are each of the following molecules capable of? For simplicity, only consider dispersion if it is the only force present or there is a large area that could interact via it alone.
Q2.3: Below are three solvents. For each molecule choose which of the three solvents would be best for maximizing solubility.

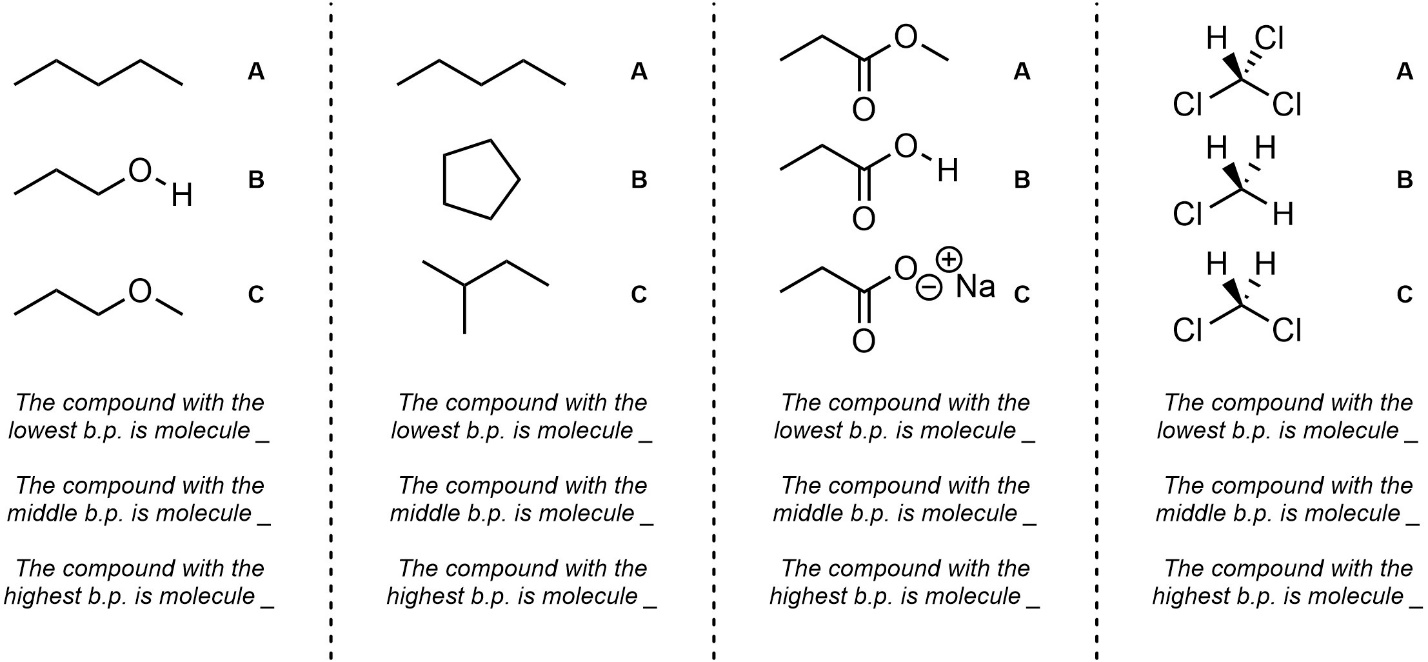
Q2.4: Rank each set of molecules in order of increasing boiling point (lowest to highest).
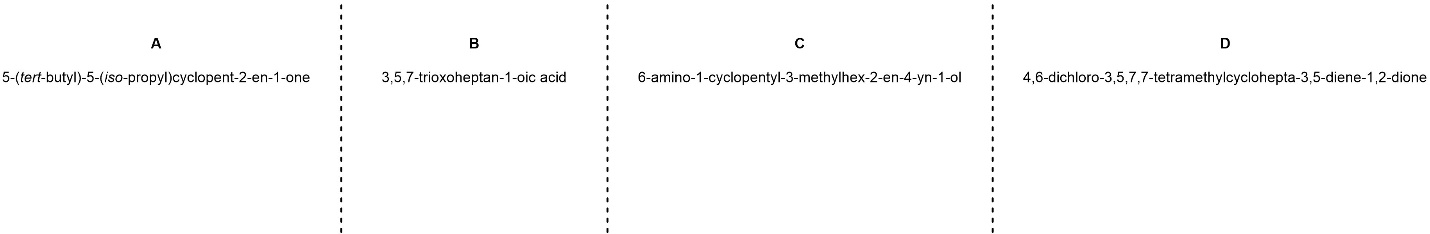
Q2.5: Name each of the following molecules using the IUPAC rules discussed in the text. Be aware that names may be (significantly) longer/shorter than the blank spaces suggest.
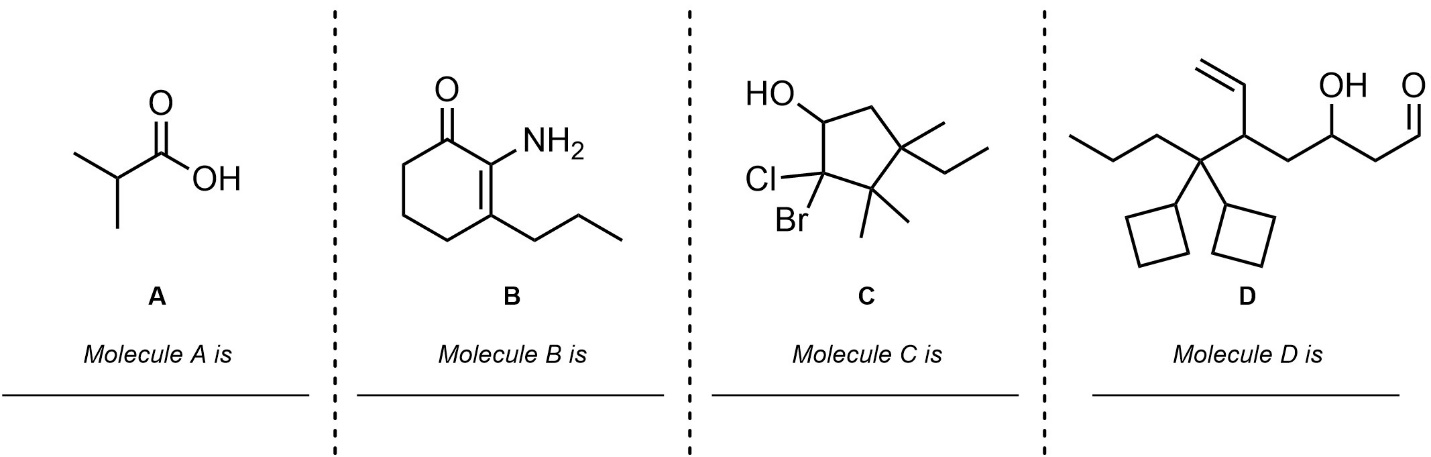
Q2.6: Draw each molecule using the provided IUPAC name.