Chapter 13 Practice Problems – Answers
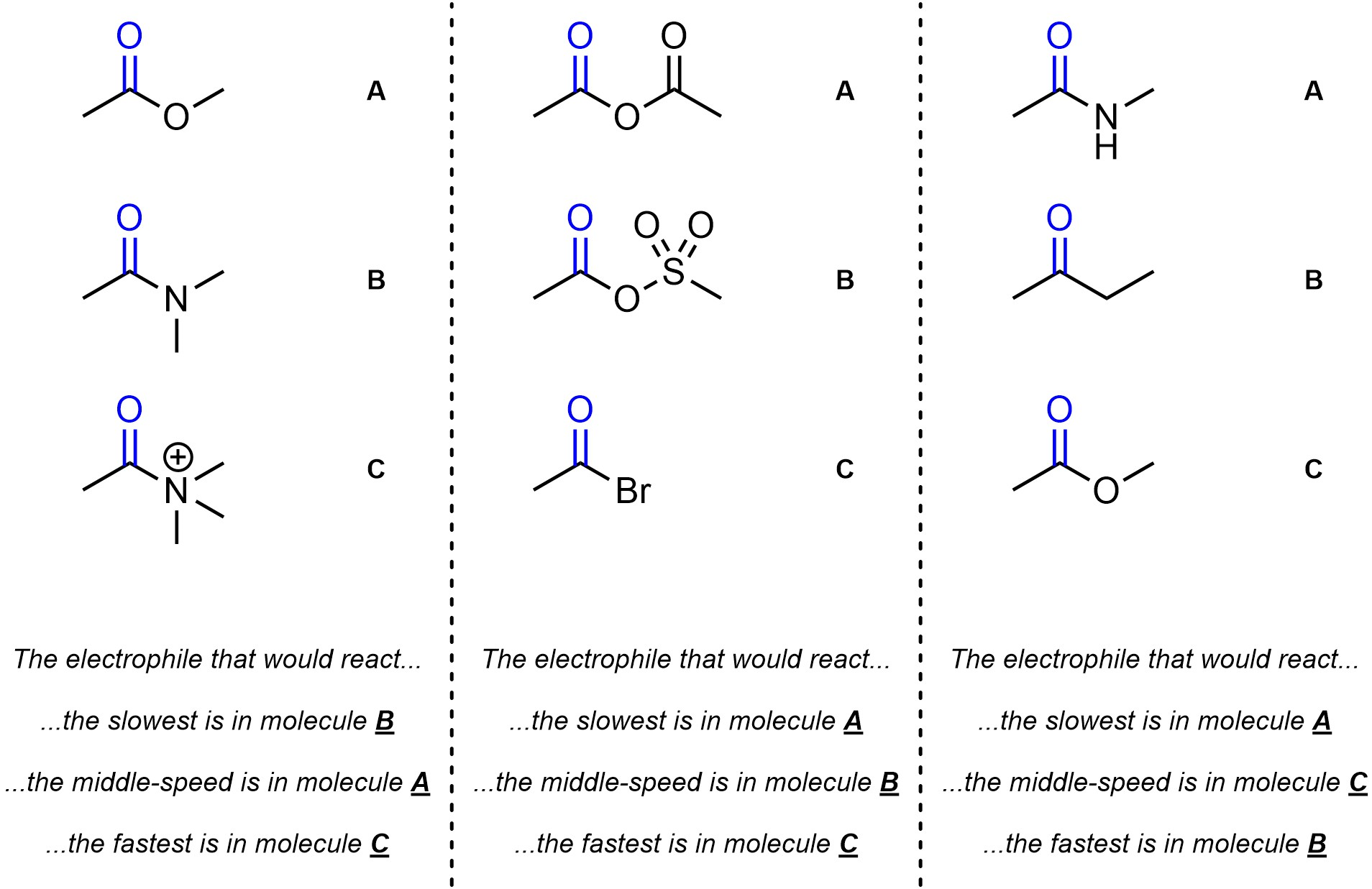
Q13.1: Rank each set of carbonyl-containing electrophiles by how quickly they would undergo addition-elimination reactions relative to each other (slowest to fastest). The carbonyls to consider are highlighted.
Ranking them as EDGs/EWGs may help.
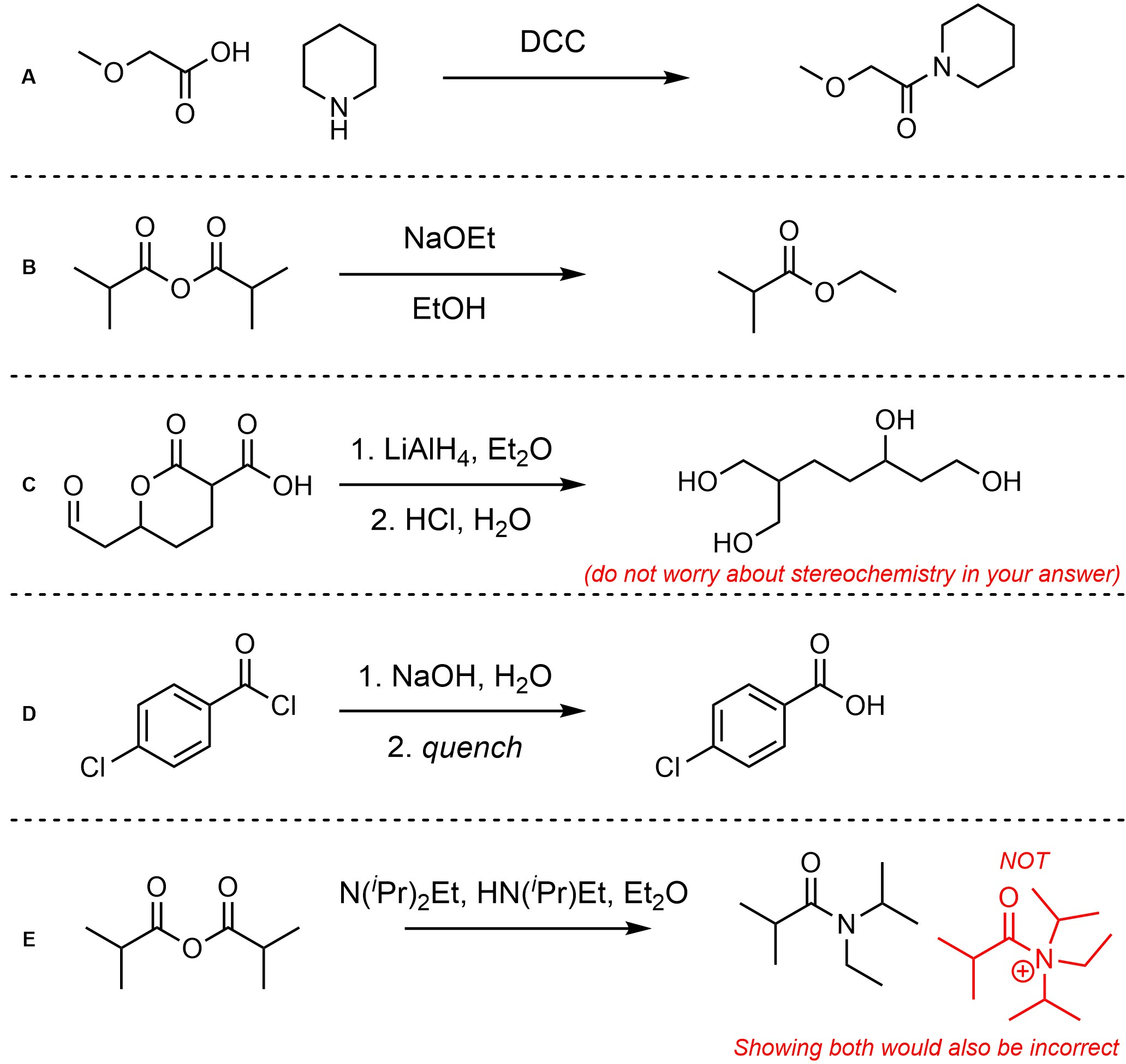
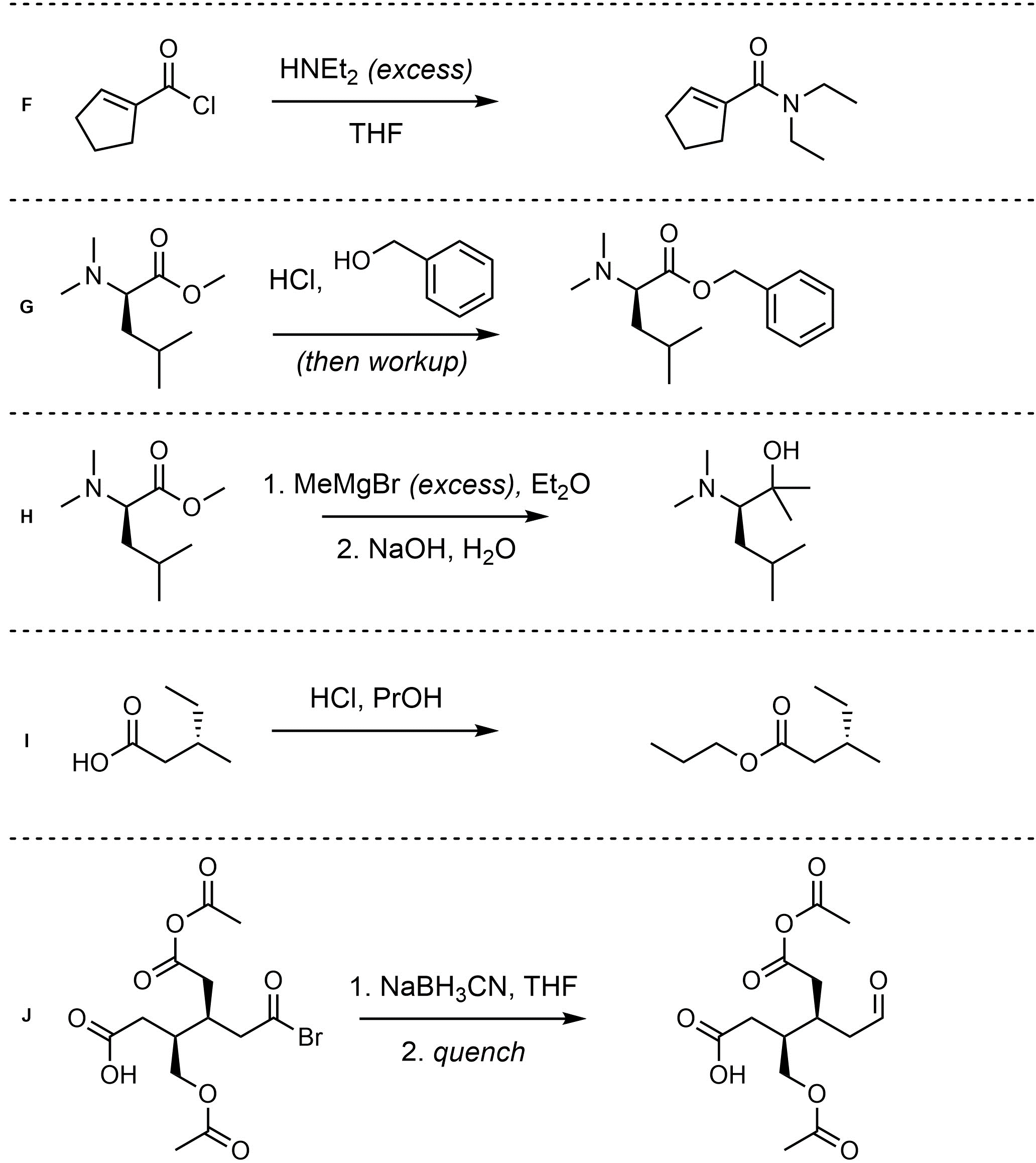
Q13.2: Assume each of the following is one (or more) addition-elimination reactions. For each reaction draw the expected major organic product(s). The products must be drawn as line-angle structures. Clearly indicate the appropriate stereochemistry using hashed and wedged bonds where necessary. If the product(s) is/are formed as more than one diastereomer, draw all diastereomers formed. If the product(s) is/are formed as a racemate (racemic mixture) draw only one enantiomer and write the word “racemic” beneath/beside it. Do not write “racemic” if it does not apply.
This chapter contains many more specific examples of chemical reactions than previous chapters.
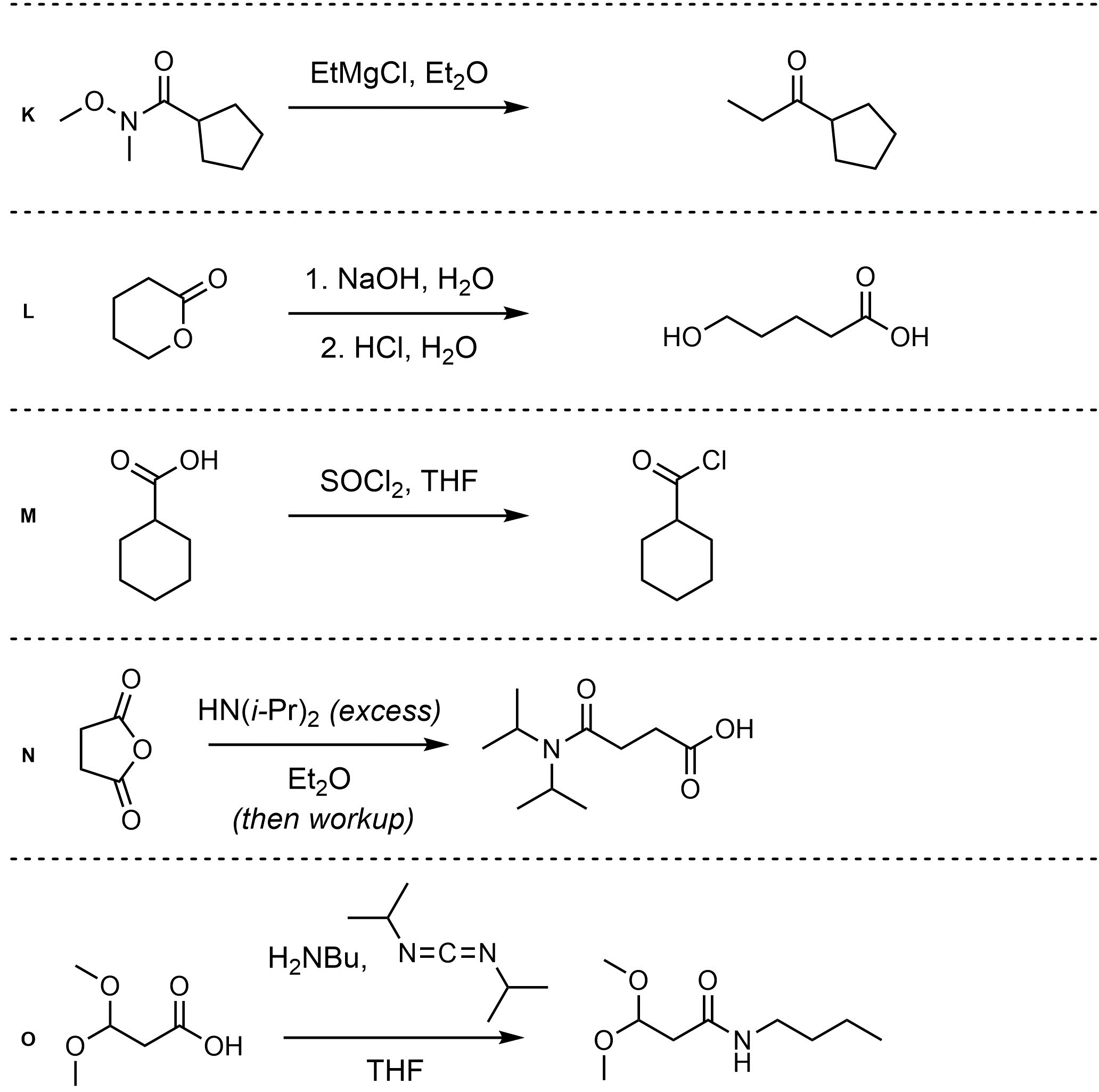
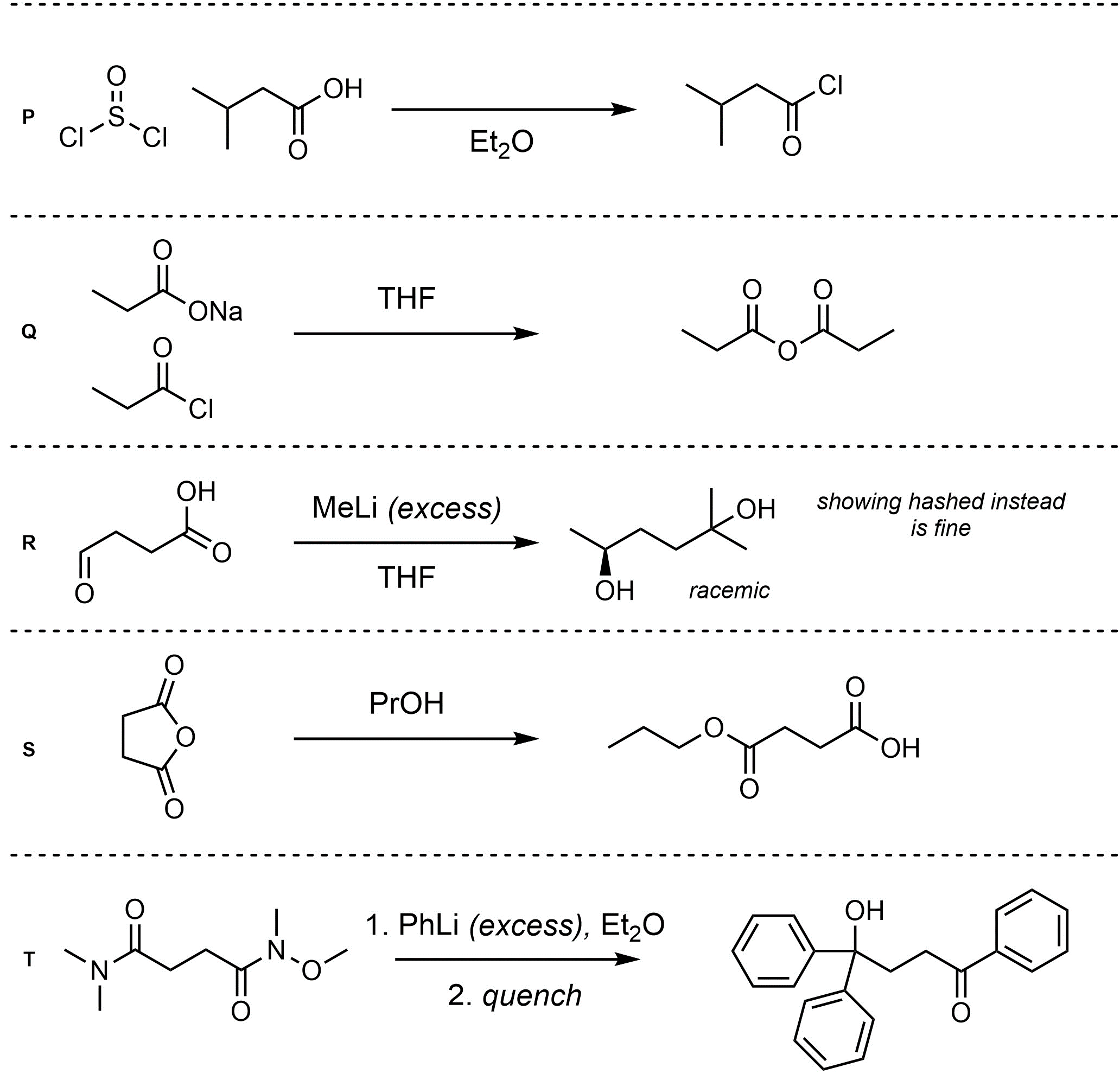
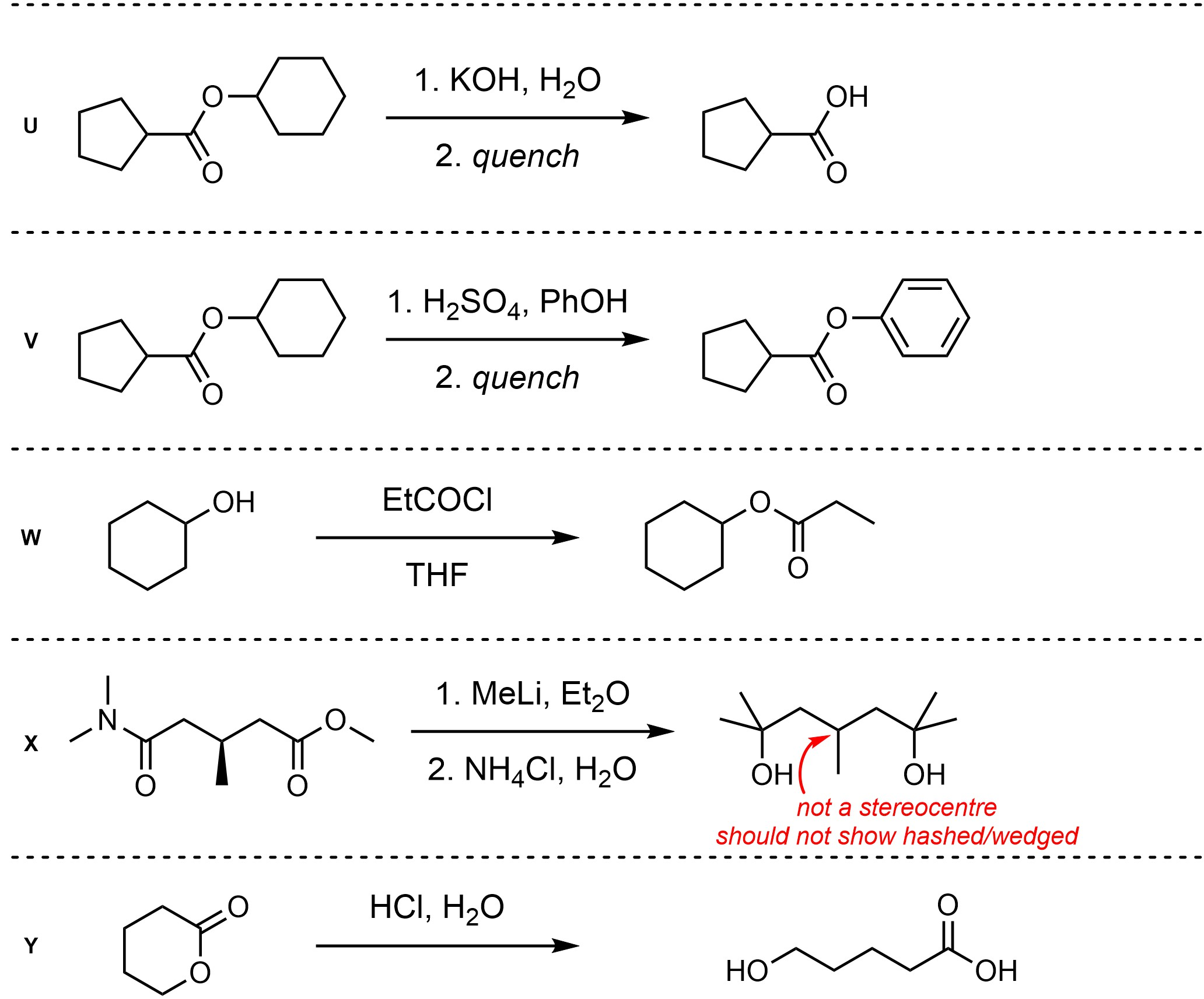
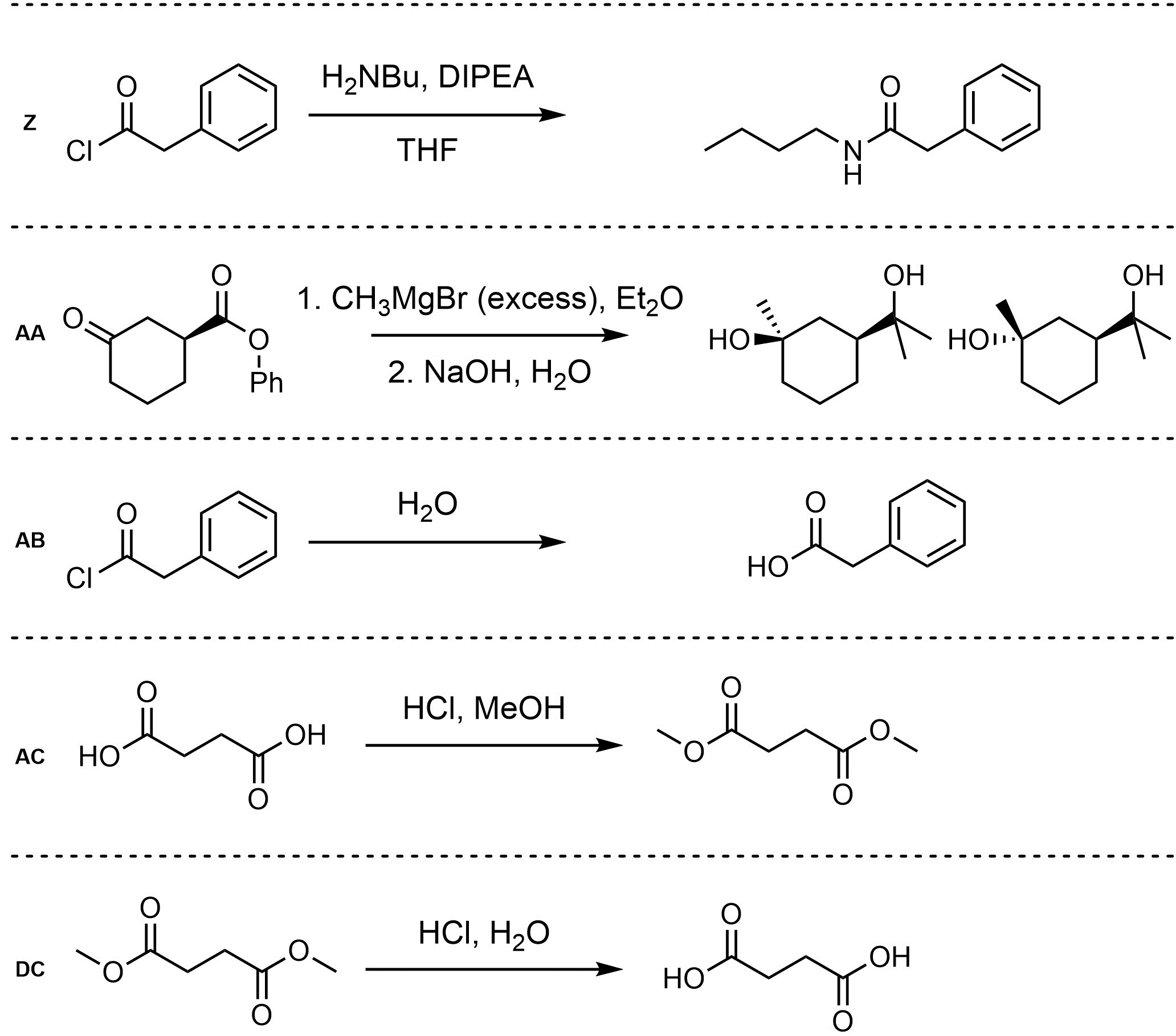
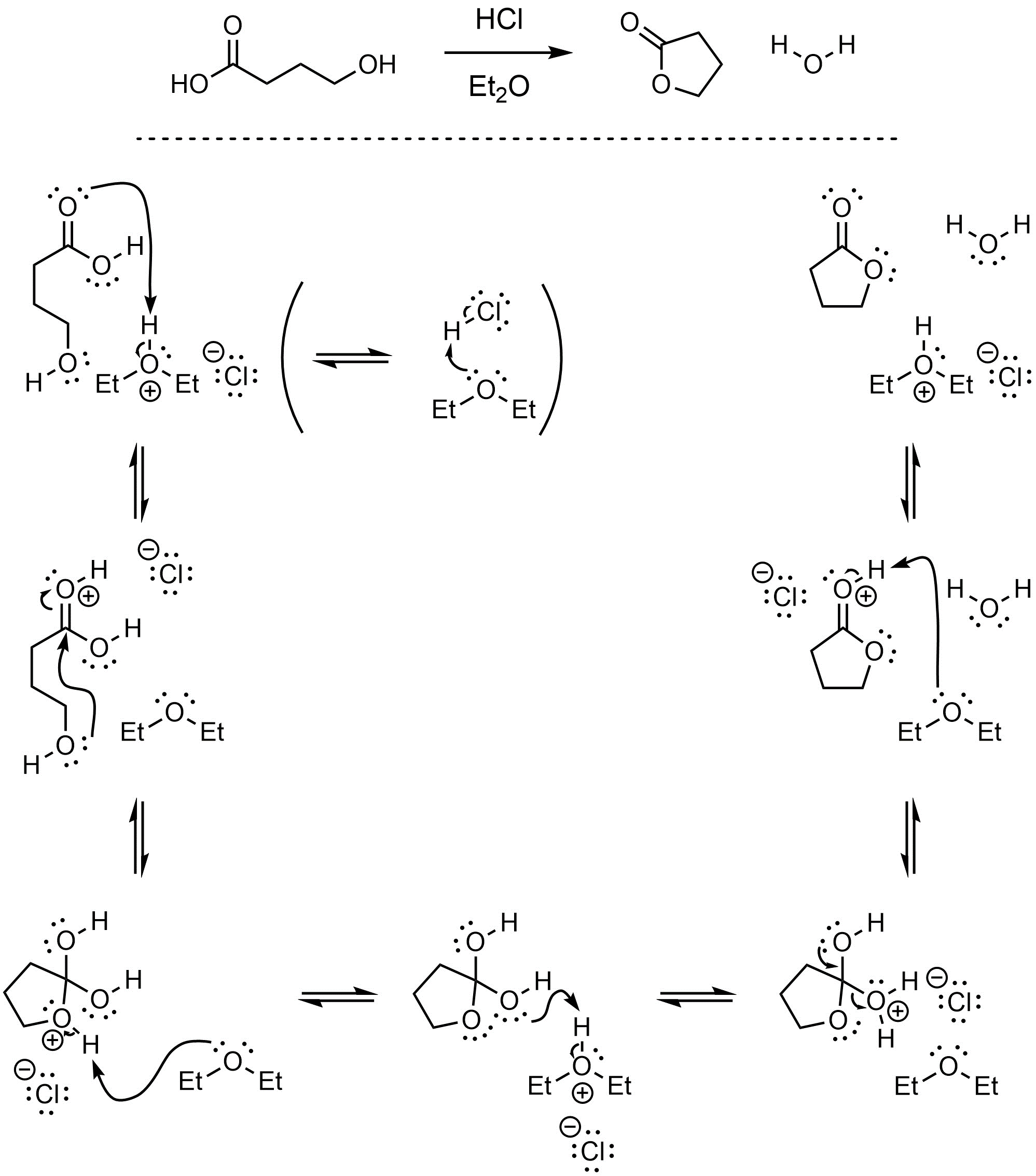
Q13.3: A reaction equation is given below. Propose a reasonable mechanism for the reaction. Show all necessary intermediates, curved arrows, lone pairs, and formal charges. If there is a catalyst remember to regenerate it at the end of the reaction.
Mechanism is the same as Scheme 13.23 in Section 13.7.2.1.
Showing HCl directly as the catalyst is acceptable but not encouraged.
Trivia:
This reaction would be performed using a desiccant or Hickman Still
to remove the water and control the equilibrium.
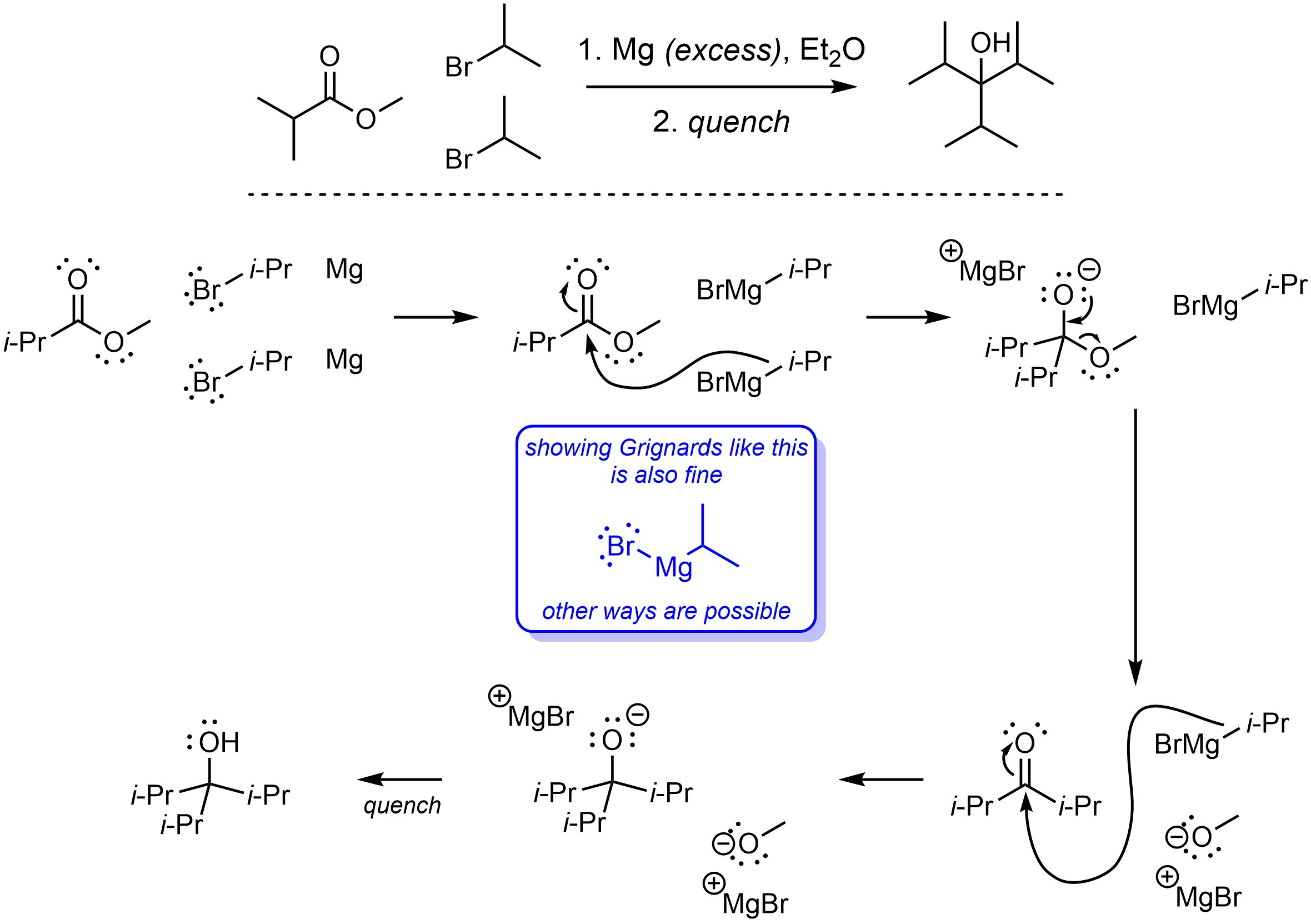
Q13.4: A reaction equation is given below. Propose a reasonable mechanism for the reaction. Show all necessary intermediates, curved arrows, lone pairs, and formal charges. If there is a catalyst remember to regenerate it at the end of the reaction. You do not need to show curved-arrows for the first mechanistic step (involving elemental magnesium) or the “quench” but you should indicate what exists before and after these steps.
Mechanism is the same as Scheme 13.43 in Section 13.9.2.
but
with a non-arrow step for formation of the Grignard compounds first.
Because the isopropyl groups are not involved in any step of the mechanism
drawing them out or abbreviating them is fine.
(could also use R = iPr)
Showing the O-Mg bonds as ionic or covalent is fine;
showing them as covalent adds a few extra curved arrows
but is straightforward to do.
There are multiple ways to draw Grignard reagents.
Any valid way is acceptable.