Chapter 10 Practice Problems – Answers
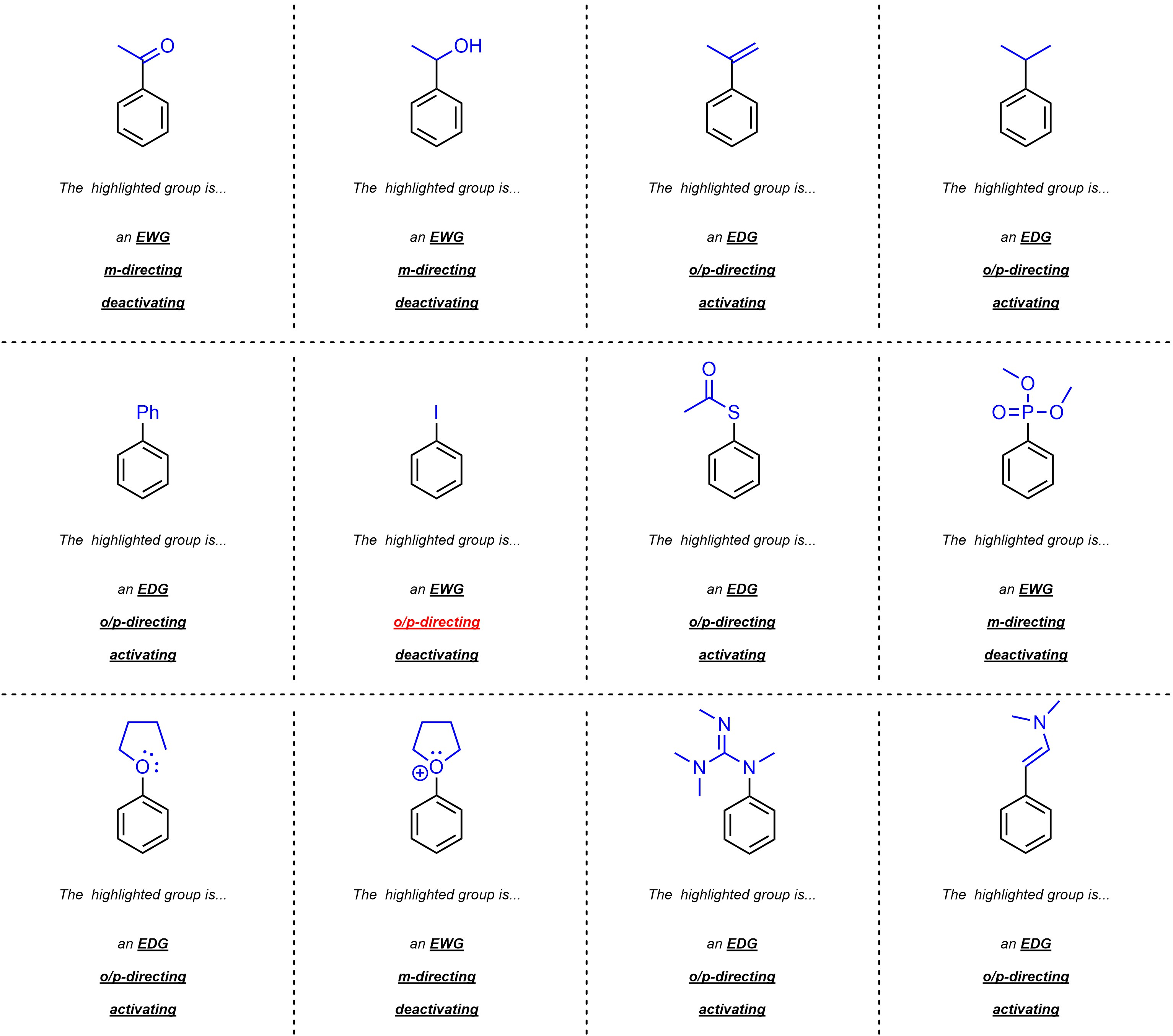
Q10.1: Class each of the following groups as an EWG or EDG; o+p-directing or m-directing; and activating or deactivating. For practice purposes you should attempt to do this without the use of a table/“cheat sheet”.
The procedure in Section 10.10.4 may help.
Remember how halides are an exception
to the standard directing and activating patterns.
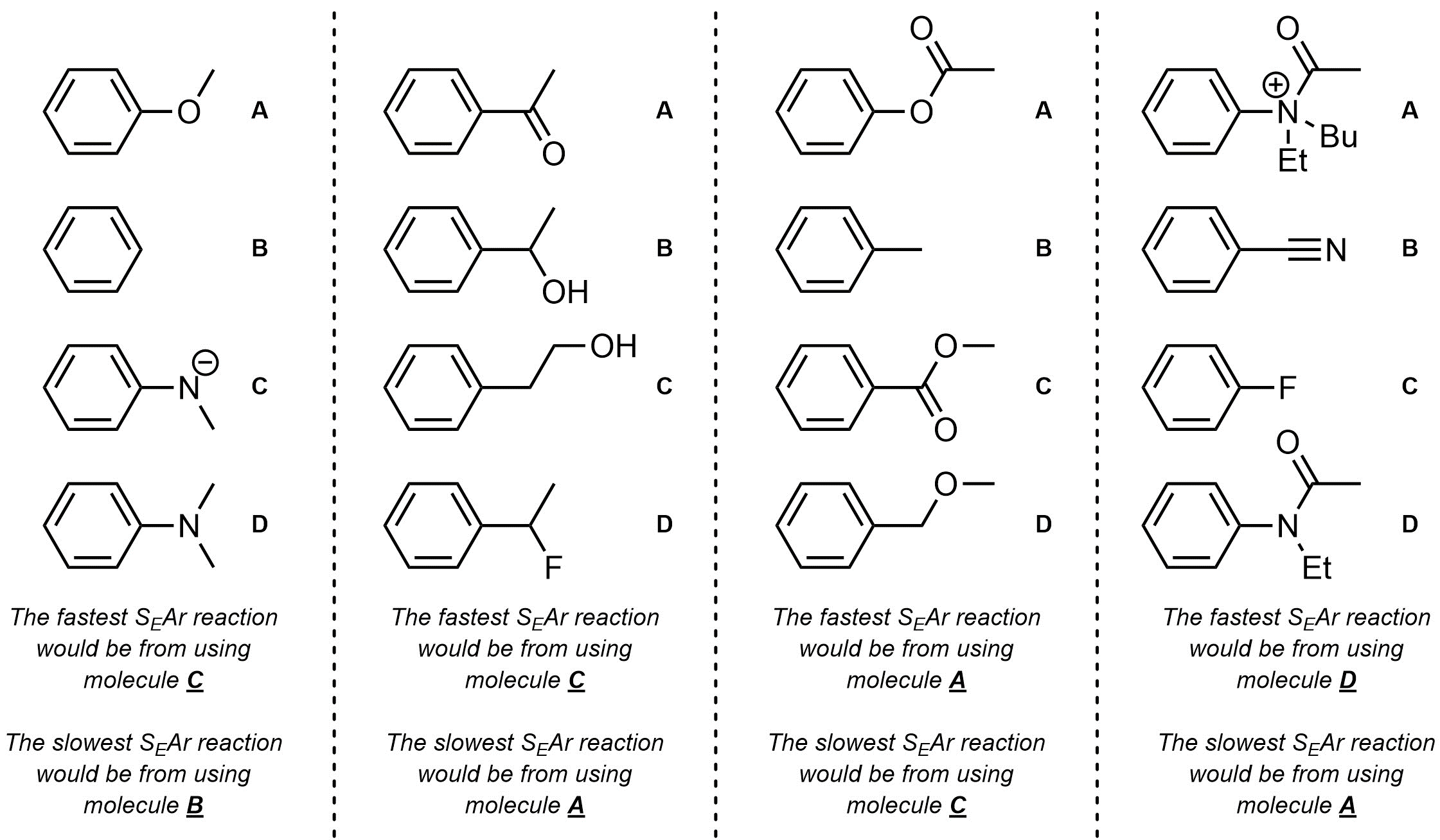
Q10.2: For each set of starting materials determine which would undergo an SEAr reaction the fastest and which would undergo an SEAr reaction the slowest.
This is comparing strength of EDG / EWG (ranking)
and thinking critically about
what effect that will have on the rate of the reaction.
Q10.3: Below is a starting material and two possible sets of reactions to transform it into the desired product. Which of the two routes will generate the product in the highest overall yield? Write and/or draw a brief explanation for why.

NOTES:
It is possible to ask a very similar question and
have different important factor(s).
It is possible to have students suggest the two steps and
require them to rationalize why they chose those steps and that order.
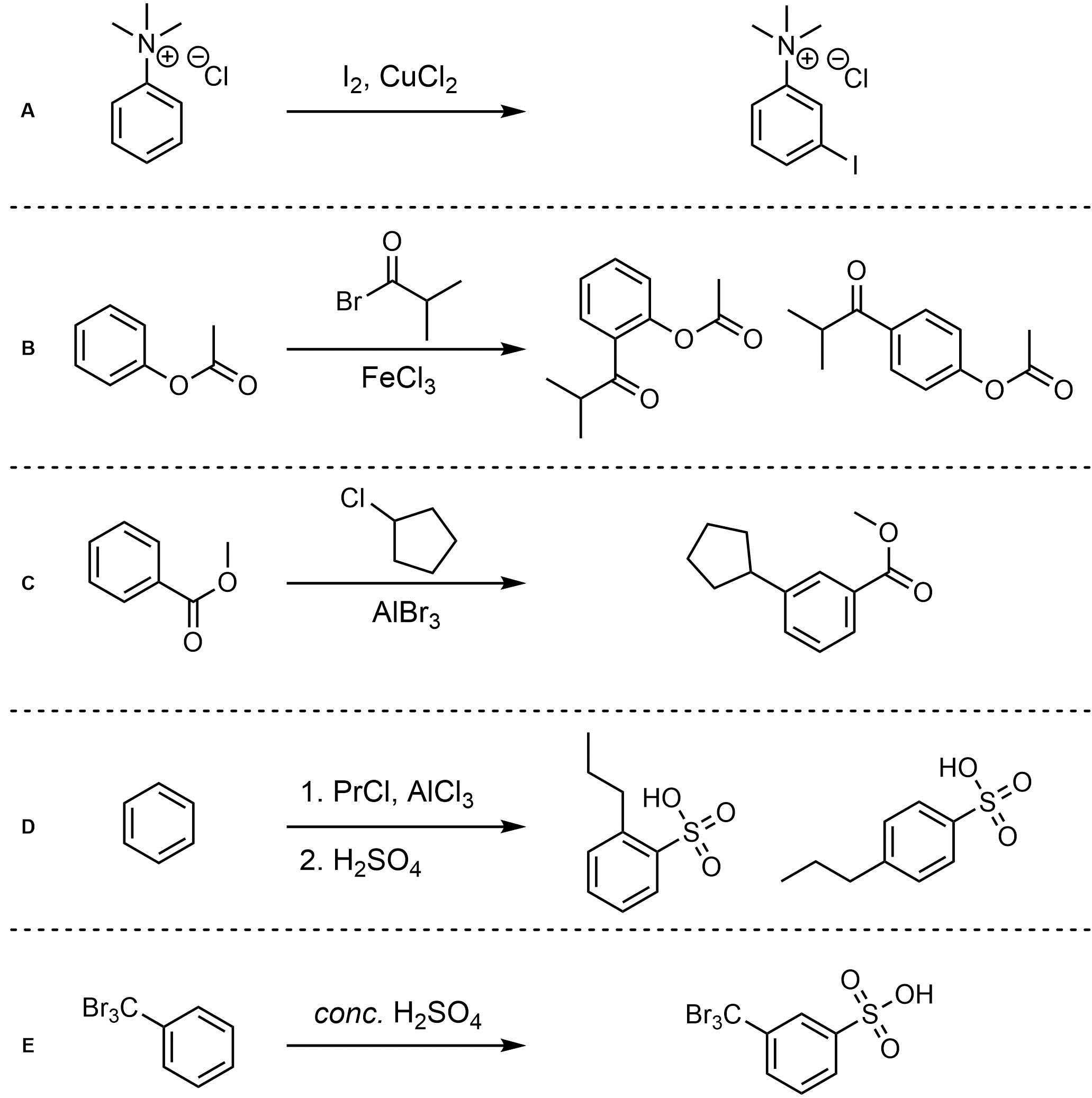
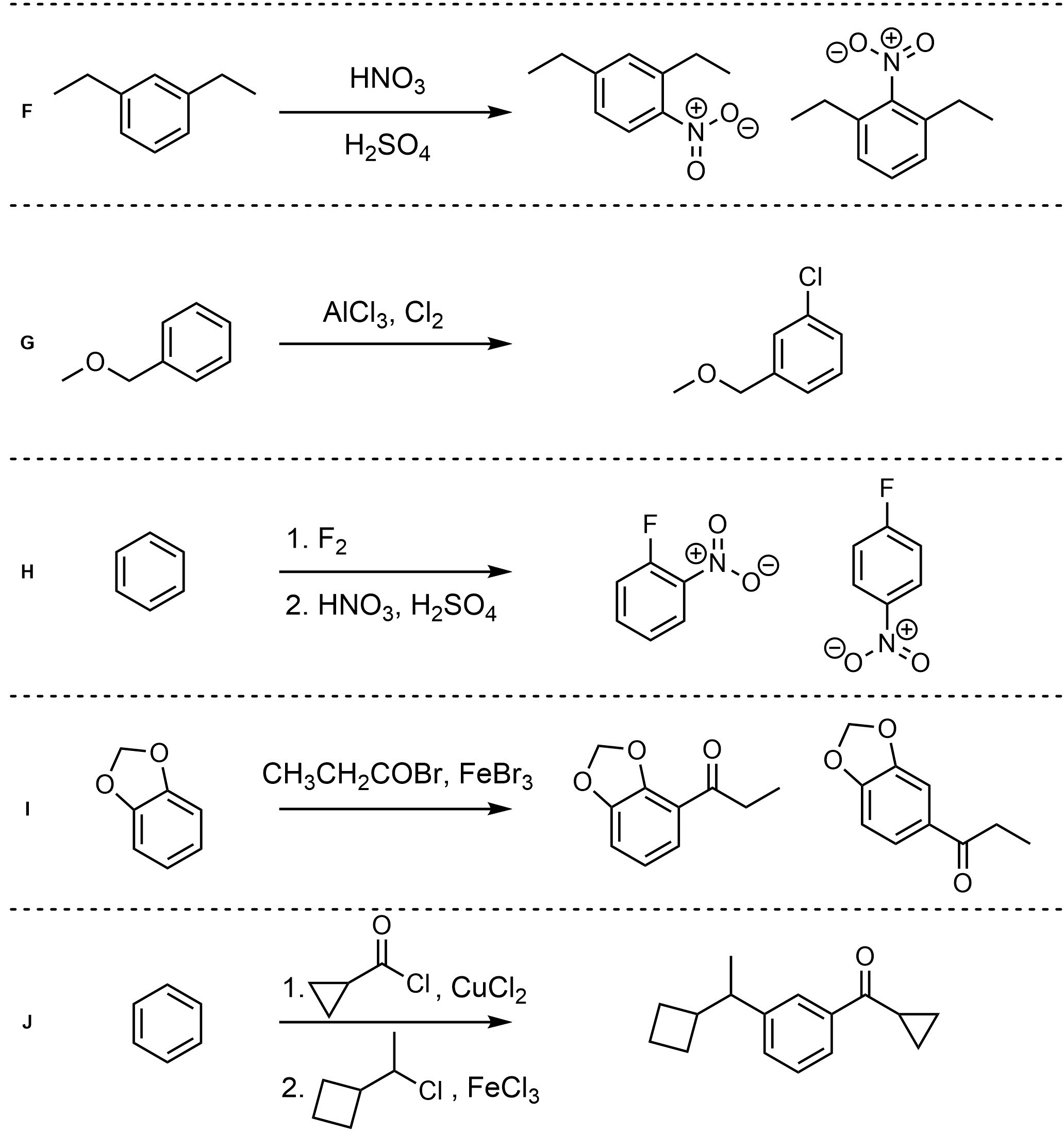
Q10.4: For each reaction draw the expected major organic product(s). The products must be drawn as line-angle structures. Assume issues such as multiple additions in Friedel-Crafts, catalyst incompatibilities, and side products do not occur.
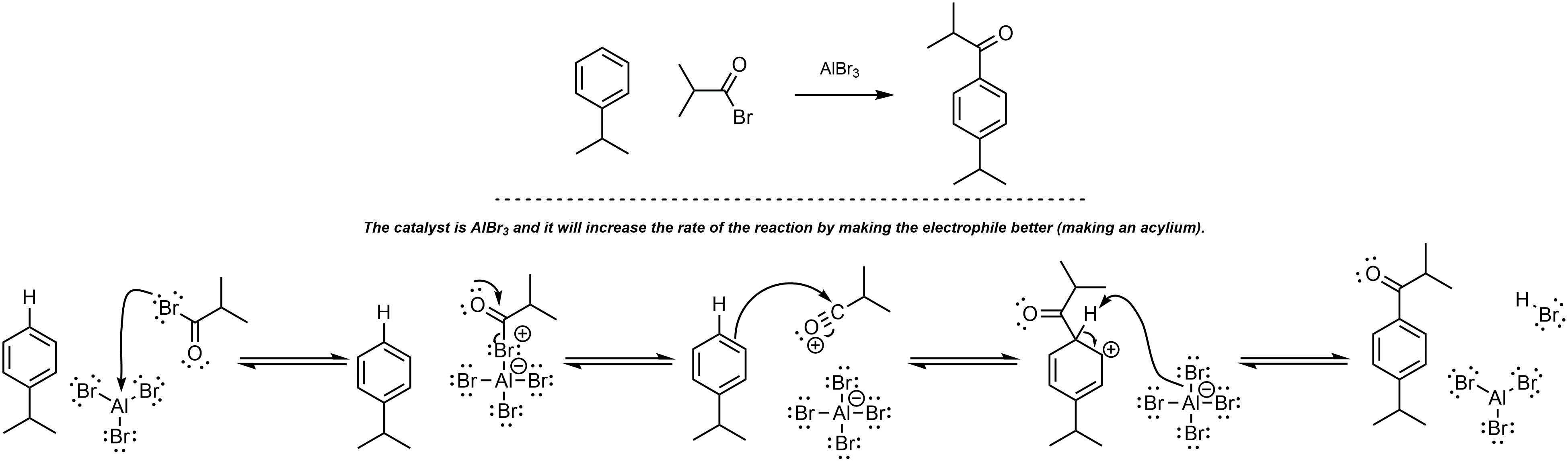
Q10.5: A reaction equation is given below. Write a brief sentence explaining which compound is the catalyst and how the catalyst will increase the reaction rate. Then propose a reasonable mechanism for the reaction. Show all necessary intermediates, curved arrows, lone pairs, and formal charges. Remember to regenerate the catalyst at the end of the reaction.
Mechanism is the same as Scheme 10.15 in Section 10.9.2
MUST generate the acylium (halide MUST leave).