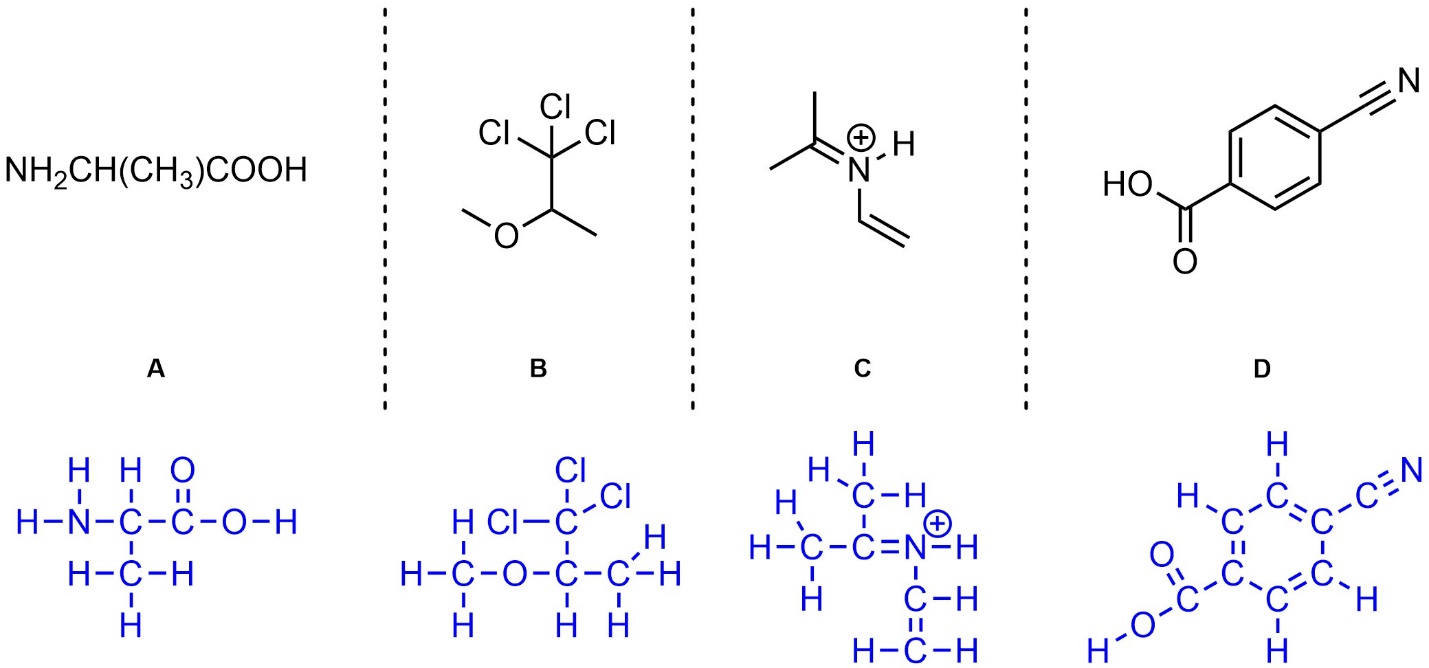
Chapter 1 Practice Problems – Answers
Q1.1: How many sigma (σ) and pi (π) bonds are there in these molecules?
Drawing in (or visualizing in your mind) implied H’s will help.
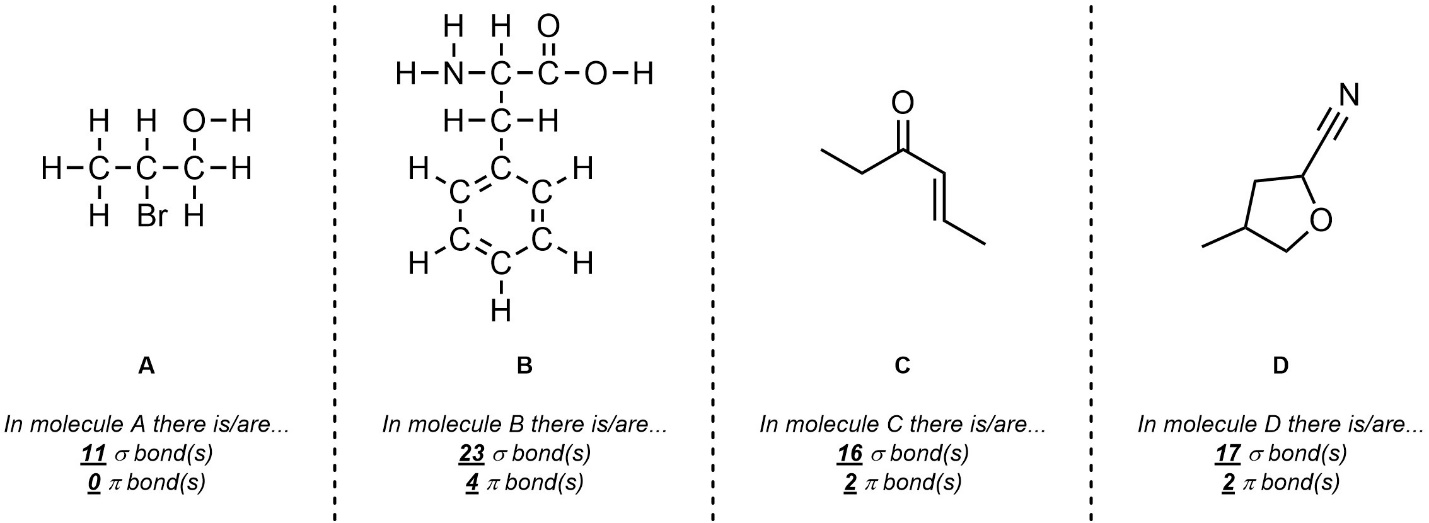
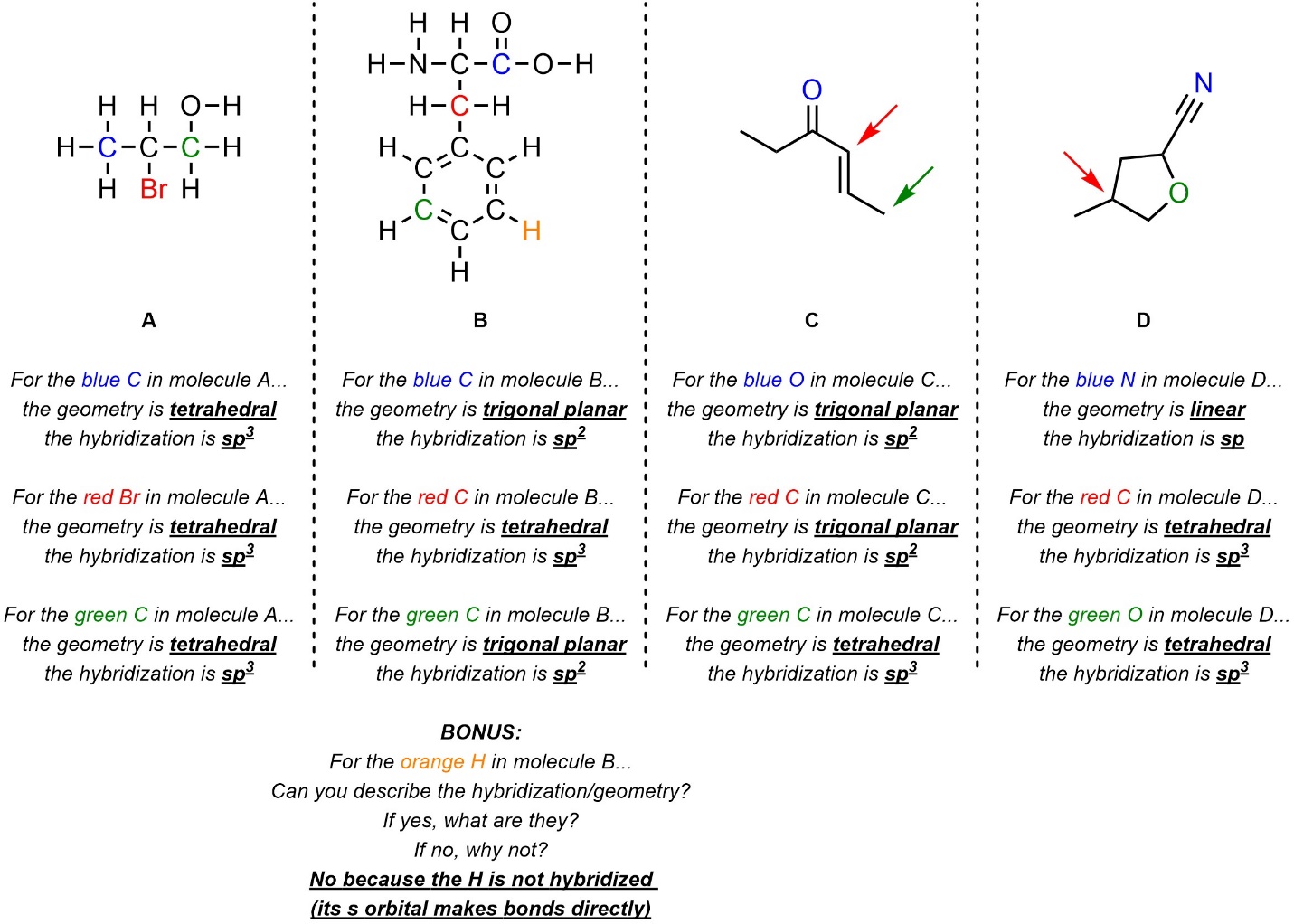
Q1.2: What is the hybridization and geometry around the highlighted atoms in these molecules? If an arrow points to a part of the molecule assume it is pointing to the carbon atom there.
Drawing in (or visualizing in your mind) implied H’s AND lone pairs will help.
Q1.3: Each molecule below contains at least one atom with a formal charge that is not being shown. For convenience all lone pairs are drawn for you. Redraw each molecule and calculate and add the missing formal charges to the appropriate places.
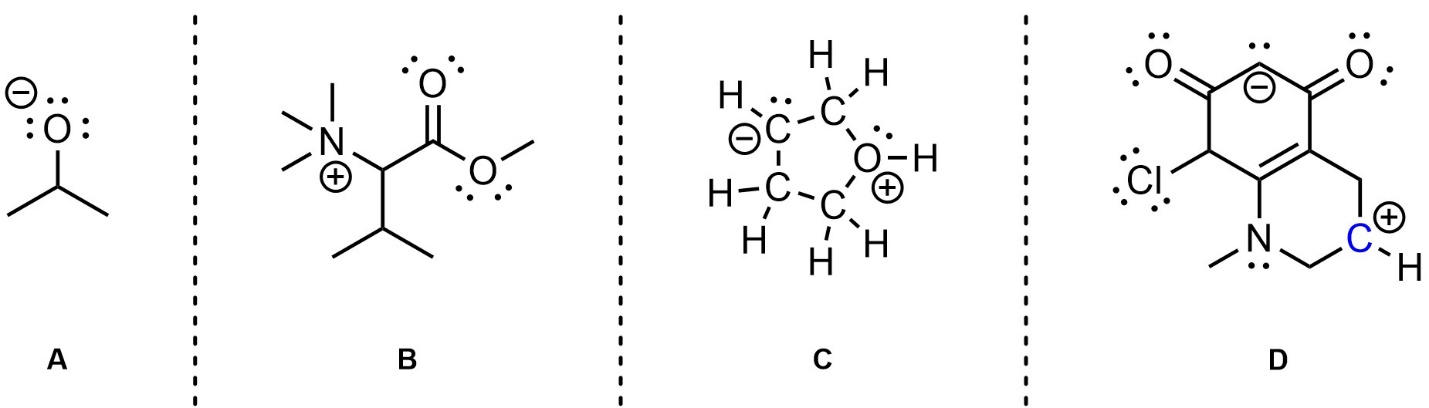
Exactly where the charges are placed
(to the left/right, above/below, etc.)
does not matter as long as it is clear which atom the charge is on.
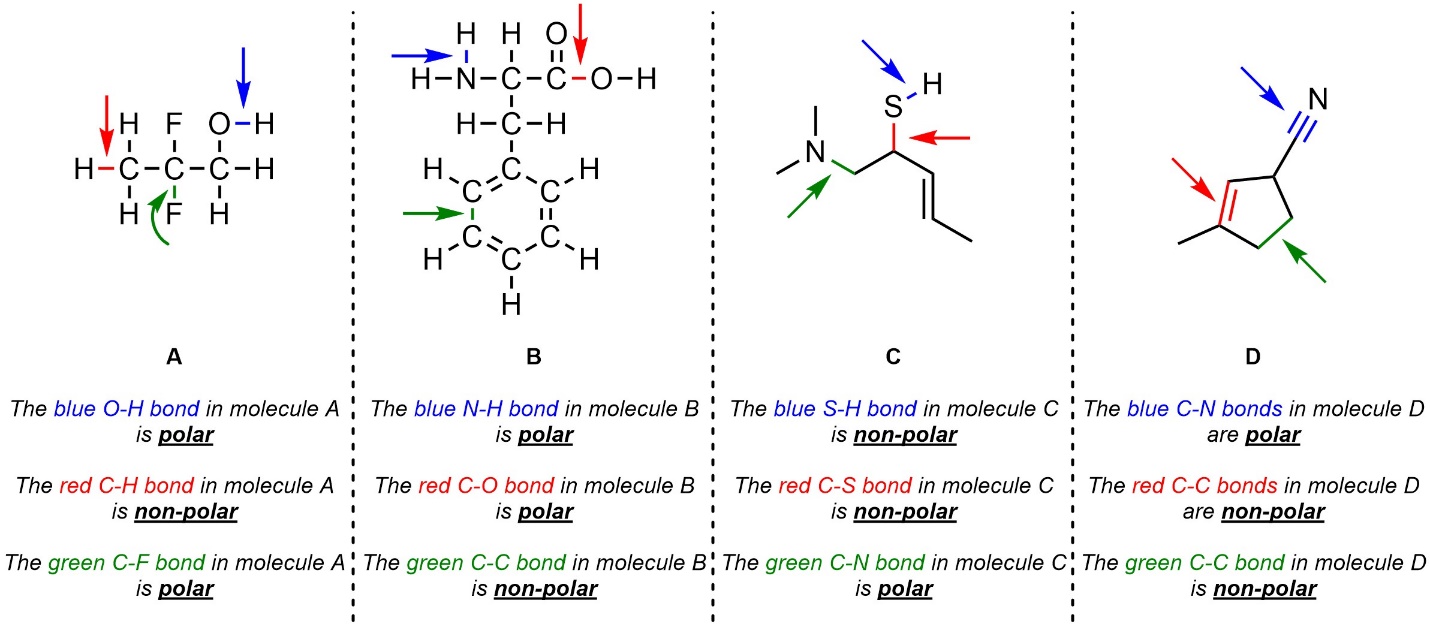
Q1.4: Class each indicated bond as polar or non-polar.
Use the Table of Electronegativities.
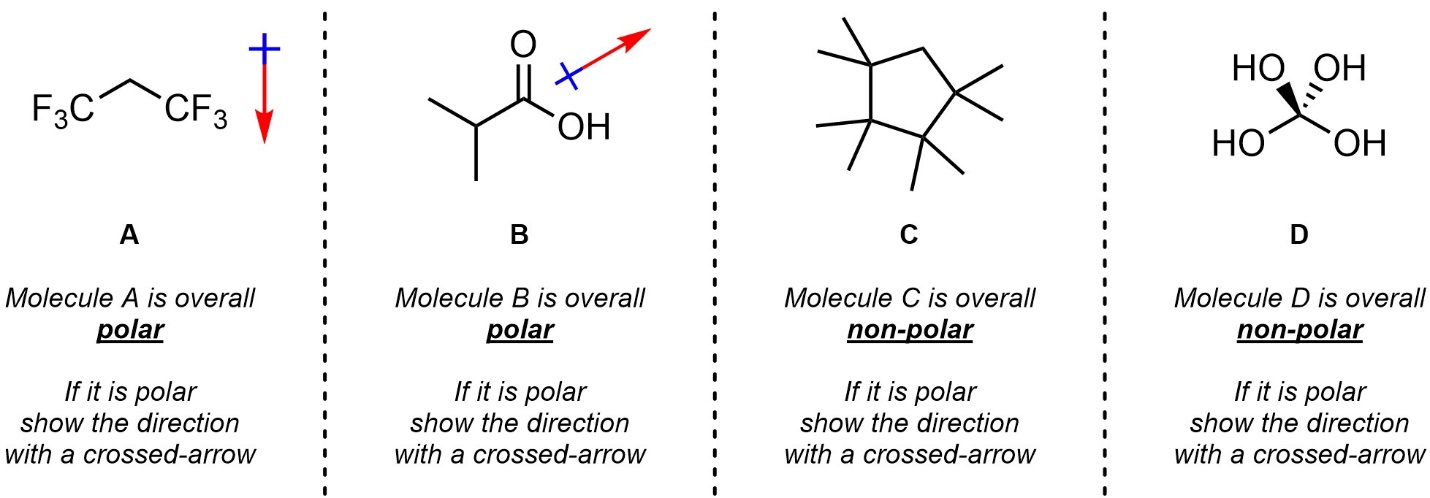
Q1.5: Class each molecule as overall polar or non-polar. If it is polar, indicate the overall direction of polarity (net dipole/dipole moment) with a “crossed arrow”.
Use the Table of Electronegativities.
Think critically about 3D geometries (especially for D).
Building a molecular model will help.
Q1.6: (Re)Draw the following as line-angle structures. Show all lone pairs.
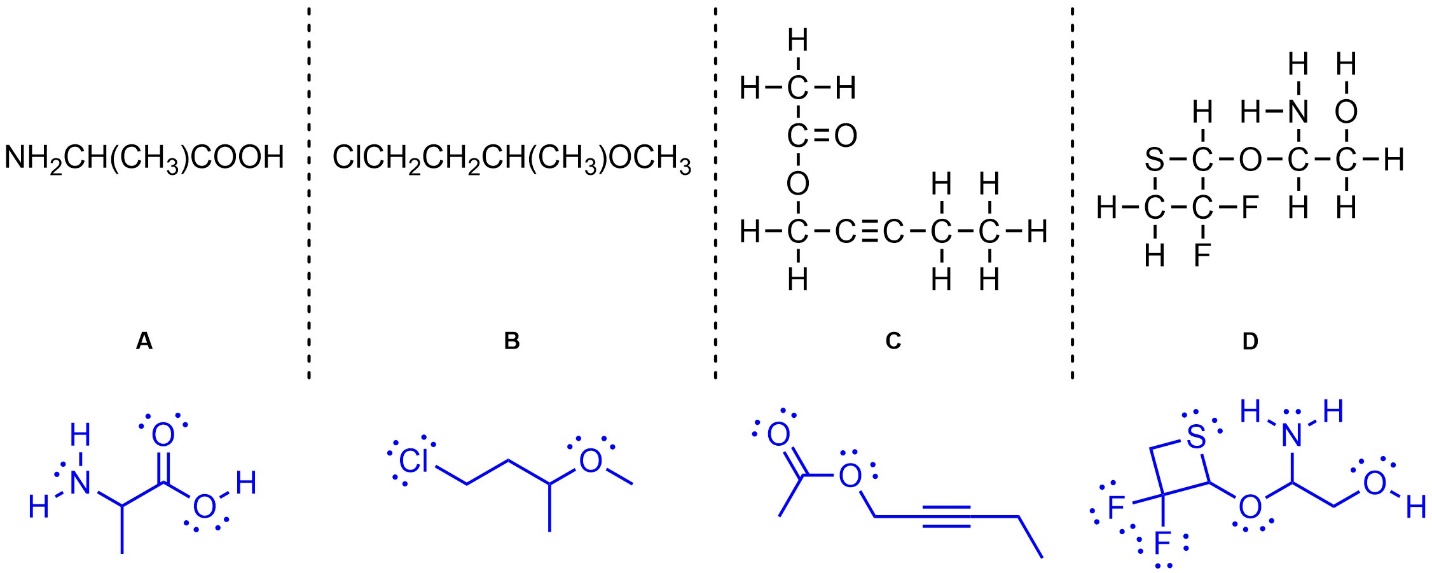
Q1.7: (Re)Draw the following as Lewis structures.
Angles should be ~90° but
bending is acceptable to avoid overlaps OR when there are rings.