8.9. Reaction: Epoxidation
Instead of adding two groups across the alkene, it is possible to add a single group connected to both carbons of the alkene. This forms a three membered ring. While many three-membered rings are unstable, epoxides (a form of cyclic ether) are not. It is possible to add oxygen (O) to both sides of an alkene, forming an epoxide (Scheme 8.29).
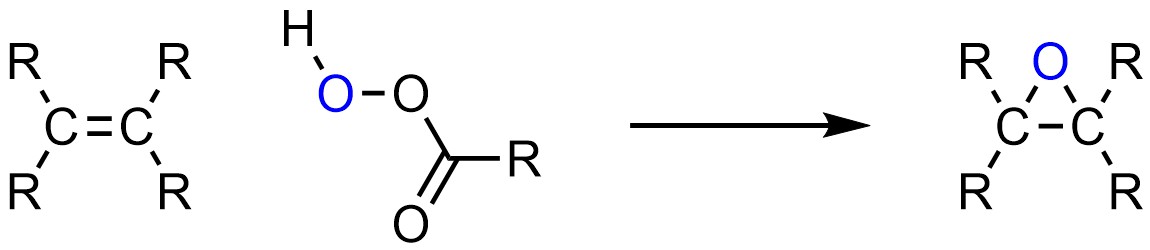
Scheme 8.29 – Generalized Reaction Equation for Epoxidation of an Alkene.
The most common reagents used to form epoxides are peroxyacids, with the overwhelming majority of reactions using meta-chloroperoxybenzoic acid (mCPBA). This is only because this compound is easily available; there is nothing special about this particular peroxyacid.
8.9.1. “Mechanism”
The currently accepted mechanism for epoxidation is complex, with 14 electrons flowing in a single step. This can be simplified/approximated by showing the movement of only 10 electrons (Scheme 8.30). Typically, having this many electrons flowing in a single reaction step would be highly unlikely. However, empirical evidence suggests this is the most accurate representation of what is occurring.
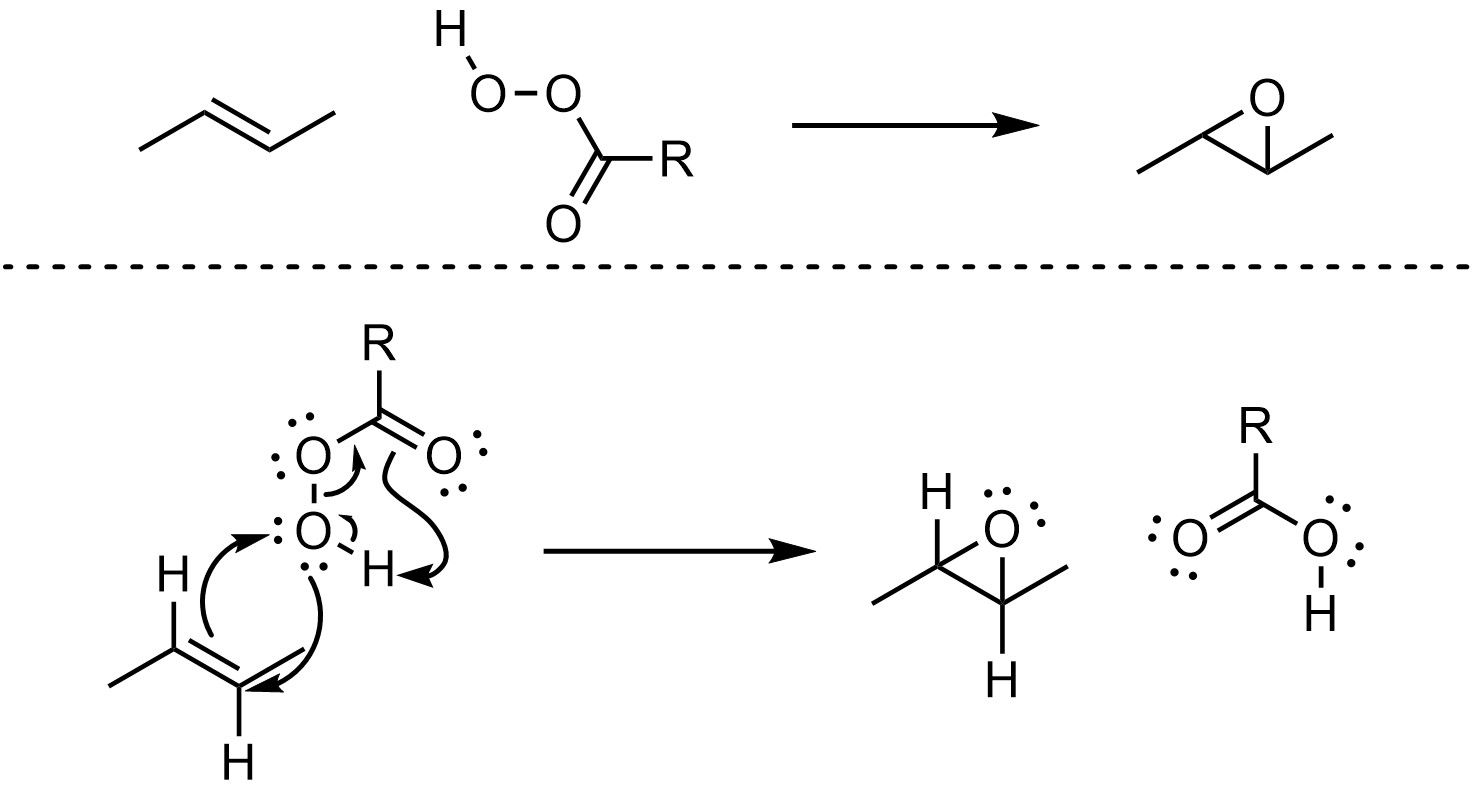
Scheme 8.30 – Approximated Reaction Mechanism for Epoxidation of the Alkene of (E)-But-2-ene.
8.9.2. Regioselectivity
Because a single group is being added to both positions, there are no regioisomers and regioselectivity is not a concern.
8.9.3. “Stereoselectivity”
Technically, epoxidations of alkenes are stereospecific (diastereospecific). The reaction forms a three-membered ring, so both C-O bonds must be above or below the previous plane of the alkene (Scheme 8.31).
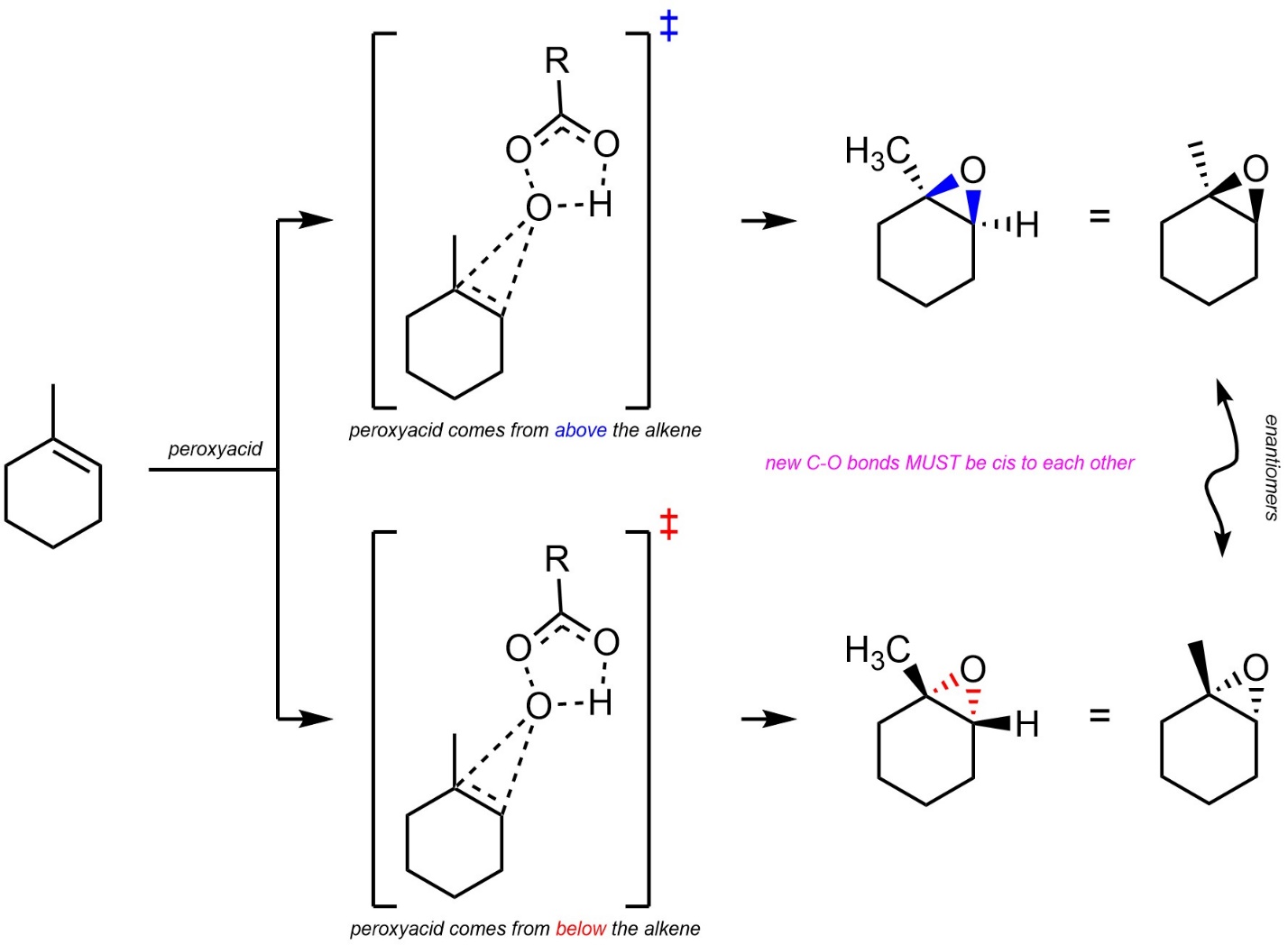
Scheme 8.31 – Diastereospecific Epoxidation of the Alkene of Methylcyclohexene.
It is very important to remember that this is a form of diastereoselectivity (by being diastereospecific). There is no enantioselectivity.

