8.8. Reaction: Addition of X-OH or X-OR
It is possible to add a halogen (X; X = F, Cl, Br, I) and an alcohol or ether (OH or OR) across an alkene (Scheme 8.25). This is what occurs if water or alcohols are present when dihalides (X2) are reacted with alkenes.
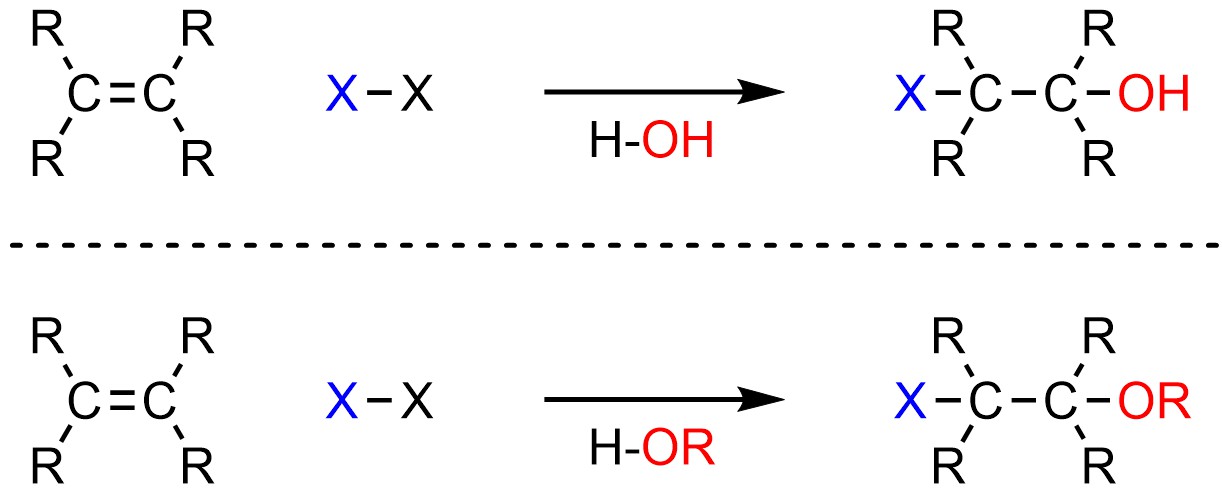
Scheme 8.25 – Generalized Reaction Equations for Addition of X-OH or X-OR Across an Alkene.
8.8.1. Mechanism and Rate-Determining Step
The mechanism for this reaction is very similar to the addition of X-X across an alkene (Scheme 8.26). Formation of the halonium occurs as before. Then, instead of the halide (a weak nucleophile) attacking the carbon, the water or alcohol (a moderate nucleophile) does. Again, the geometry of the halonium forces a stereospecific addition. Finally, another equivalent of water/alcohol removes a proton, generating the final product.
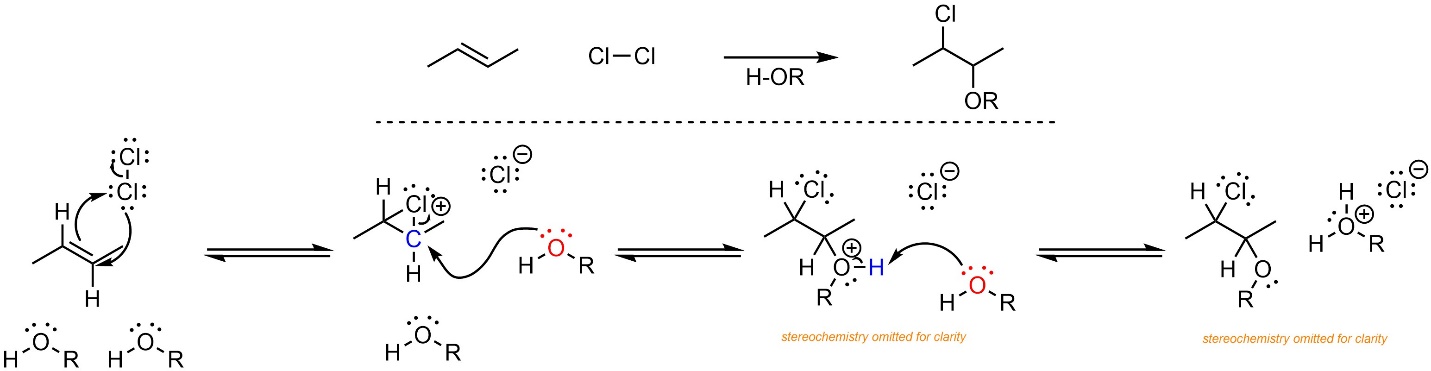
Scheme 8.26 – Reaction Mechanism for Addition of Cl-OR Across the Alkene of (E)-But-2-ene, Stereochemistry Omitted.
Although no carbocation is formed, a high-energy halonium is only slightly less energetic. The rate-determining step is the formation of the halonium. The overall reaction is exergonic.
8.8.2. Regioselectivity
As before, the major regioisomer will be the Markovnikov product. This is due to the development of a partial cationic charge on the carbon atom during the transition state, which has a similar effect to an energy difference in carbocation intermediates (Scheme 8.27).
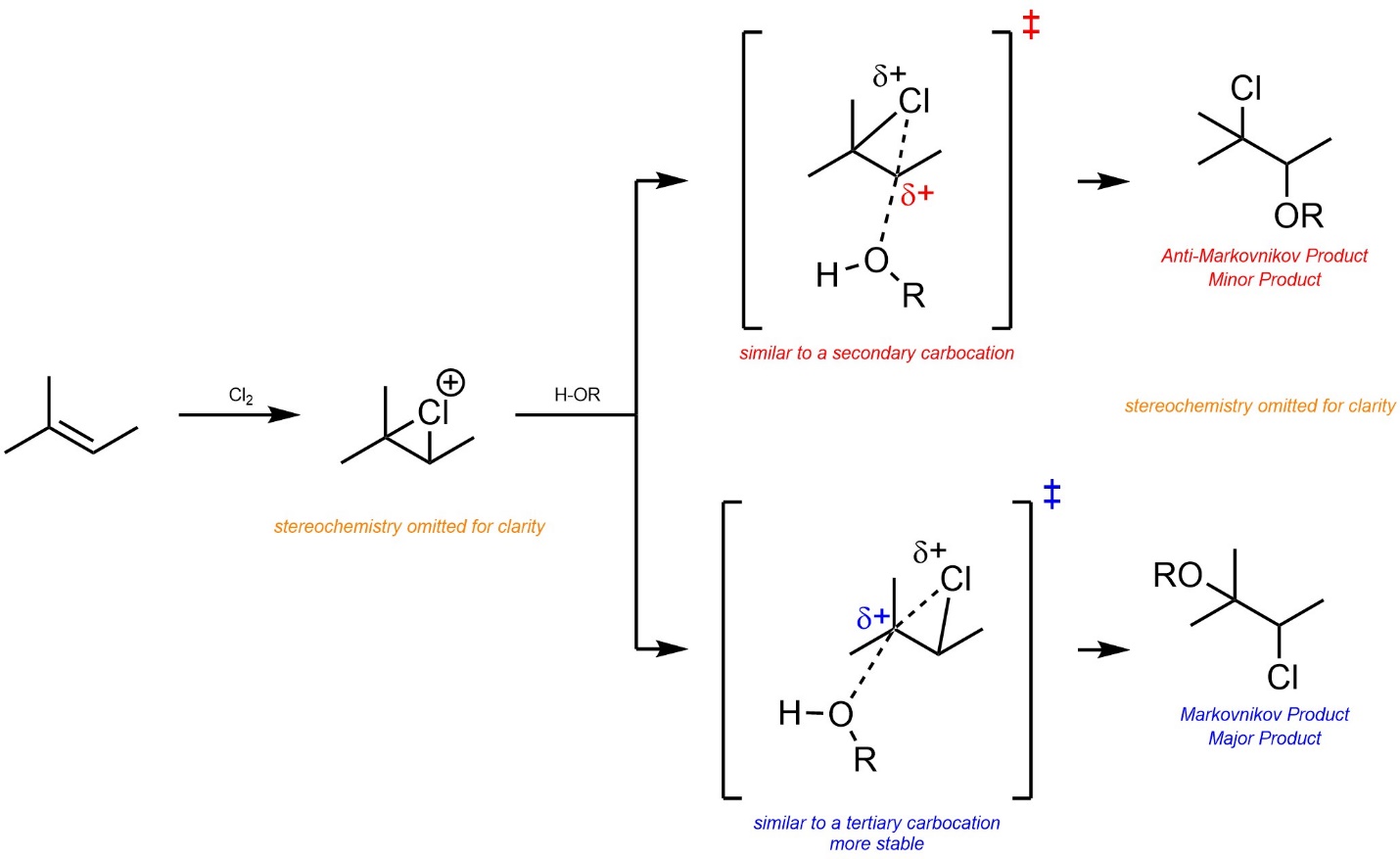
Scheme 8.27 – Determination of Major Product (Regioisomer) for Addition of Cl-OR Across the Alkene of 2-Methylbut-2-ene, Stereochemistry Omitted.
This could also be demonstrated using overlapped reaction coordinates for the two pathways.
8.8.3. Stereoselectivity – Stereospecific: trans
Additions of X-OR across alkenes are stereospecific (diastereospecific). All stereoselectivity considerations for this reaction are identical to those for the addition of X-X across an alkene (see Section 8.7.3.). It is only possible for the water/alcohol to add to the carbon by attacking from the opposite side of the halonium (trans addition; Scheme 8.28).
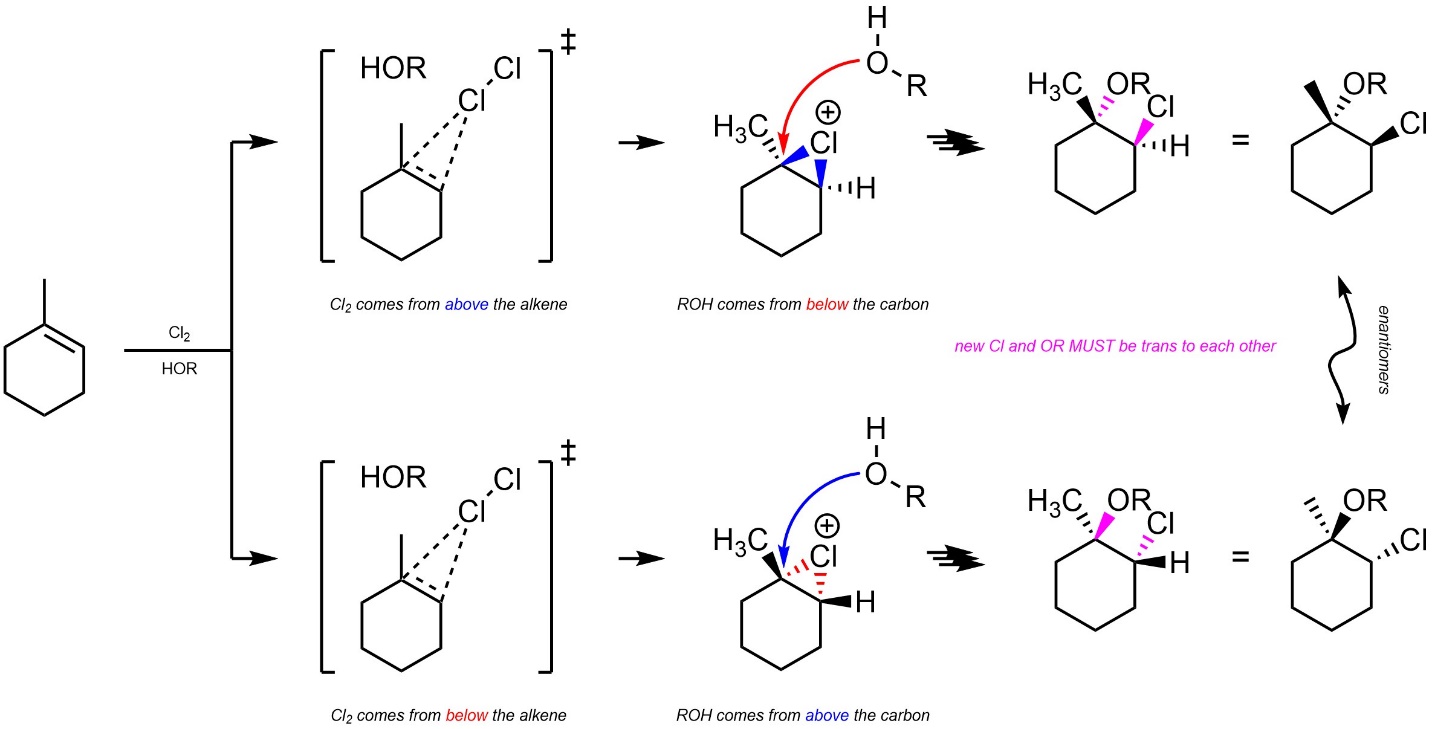
Scheme 8.28 – Diastereospecific Addition of Cl-OR Across the Alkene of Methylcyclohexene.
It is very important to remember that this is a form of diastereoselectivity (by being diastereospecific). There is no enantioselectivity.

