7.5. Catalysis of Addition Reactions
Catalysis is a broad field of study focused on improving the speed of chemical reactions. Catalysis may allow some reactions which could not otherwise occur take place. The general idea is to use some compound, which is not consumed in the reaction, to either make an energetically impossible reaction possible or make a slow reaction faster than it would otherwise be.
A common misconception is that catalysis accelerates reactions by lowering the activation energy. This is effectively true, but inaccurate. More specifically, catalysis provides an alternative pathway for the reaction to proceed through. This means that the transition states and intermediates are different from the uncatalyzed reaction, and these different species have different (lower) activation energies (Figure 7.12). Additional steps in the pathway (and new intermediates and transition states) are often added. Recall that the number of steps in the reaction does not affect the overall reaction rate. For example, a reaction with one step (one transition state) can be slower than a reaction with fifty steps.
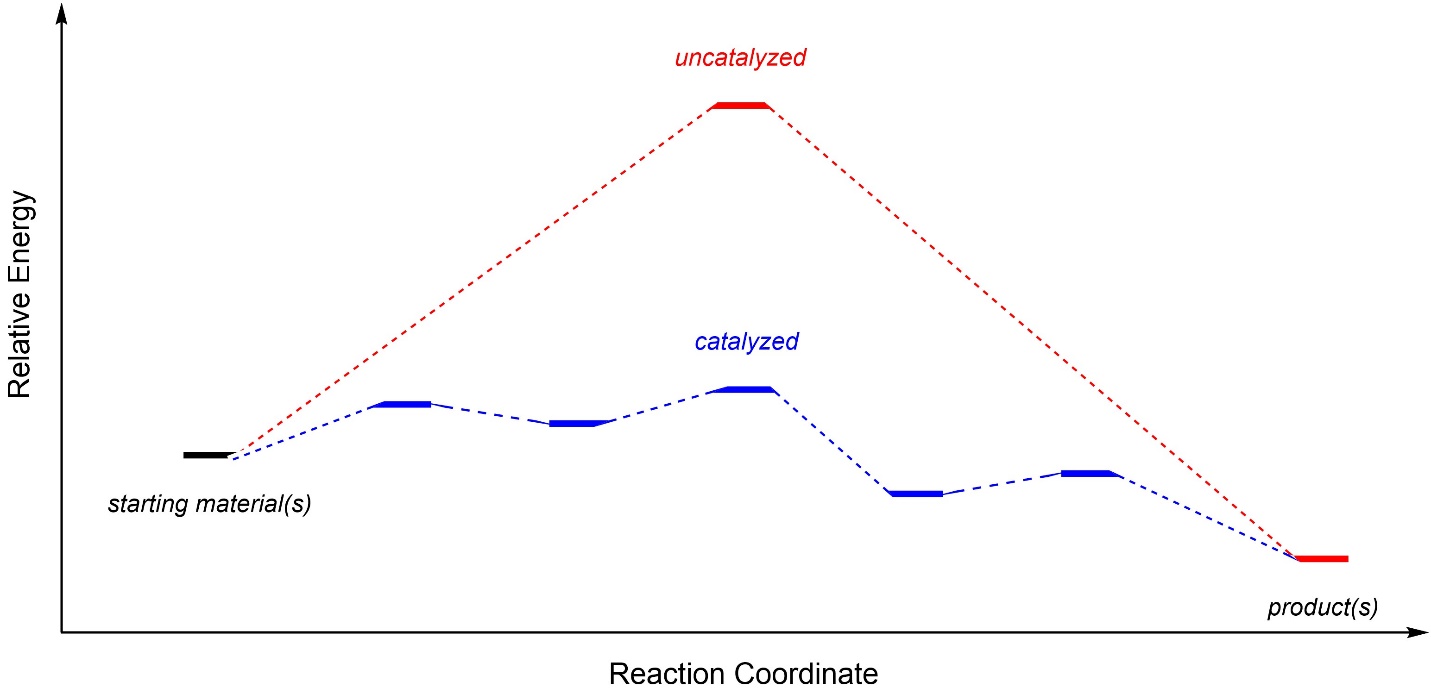
Figure 7.12 – Comparison of Uncatalyzed and Catalyzed Reaction Coordinates for a Hypothetical Reaction.
Catalysis only affects the overall speed of reactions (kinetics), it does not change the relative energies of the starting materials and products (thermodynamics). As a result, catalysis does not affect equilibrium ratios (the relative amounts of starting materials and products at equilibrium). A system may be able to reach equilibrium faster through catalysis, but it cannot be biased towards making more or less of the product than equilibrium would allow via catalysis alone.
When proposing mechanisms that involve catalysis it is important to always regenerate the catalyst at the end of the mechanism. If a proposed mechanism for a catalyzed reaction does not regenerate the catalyst at the end, it is incorrect.
7.5.1. Base Catalysis in Addition Reactions – Improving the Nucleophile
Addition reactions are commonly catalyzed by either acids or bases. When base catalysis is used the nucleophile becomes anionic, which increases the amount of electron density and, by extension, the nucleophilicity; base catalysis works by making the nucleophile stronger.
For example, water and carbonyl-containing compounds like acetone can undergo an addition reaction to form hydrates (Scheme 7.7). The uncatalyzed mechanism relies on water, a weak to moderate nucleophile, attacking the ketone, a moderate electrophile. As a result, this reaction is moderately slow. When base catalysis is used the nucleophile switches from being water to hydroxide, a strong nucleophile. As a result, the base-catalyzed reaction is much faster.
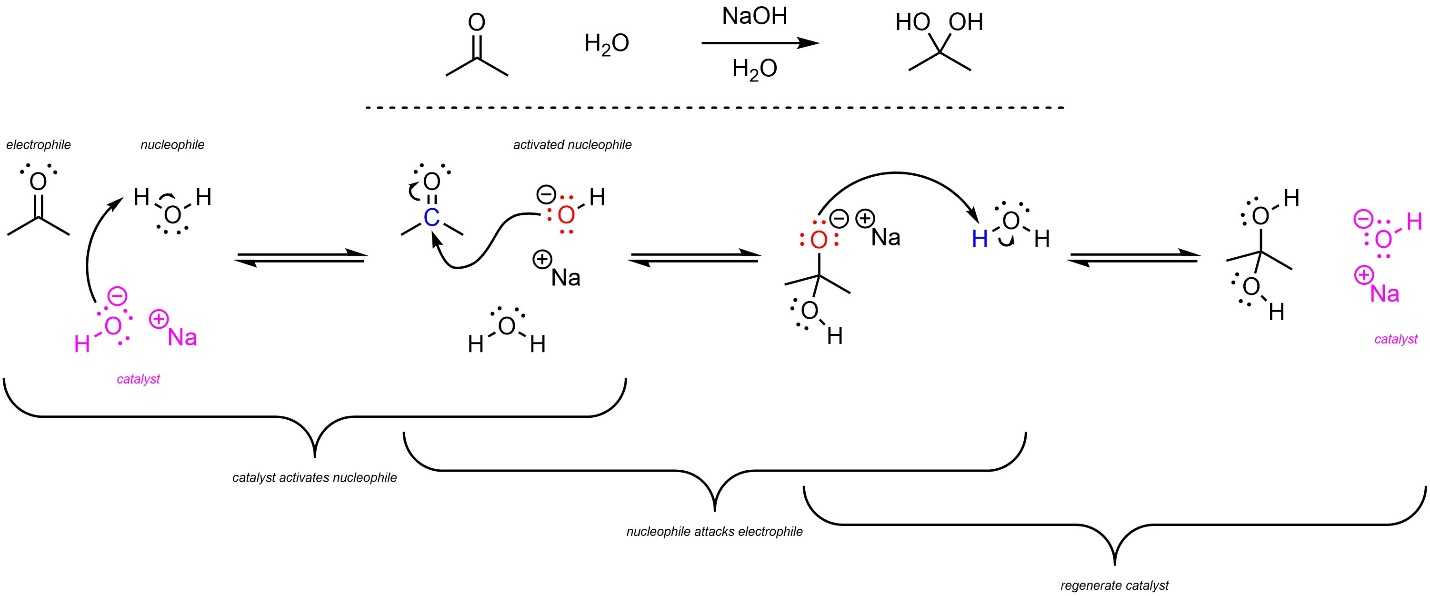
Scheme 7.7 – Reaction Mechanism for Water Attacking Acetone with Base Catalysis.
7.5.2. Acid Catalysis in Addition Reactions – Improving the Electrophile
Addition reactions are commonly catalyzed by either acids or bases. When acid catalysis is used the electrophile becomes cationic, which decreases the amount of electron density and, by extension, increases the electrophilicity; acid catalysis works by making the electrophile stronger.
For example, carbonyl-containing compounds like acetone can undergo an addition reaction with water to form hydrates (Scheme 7.8). The uncatalyzed mechanism relies on water, a weak to moderate nucleophile, attacking the ketone, a moderate electrophile. As a result, this reaction is moderately slow. When acid catalysis is used the electrophile switches from being a ketone to a protonated ketone (a type of oxonium), a strong electrophile. As a result, the acid-catalyzed reaction is much faster.
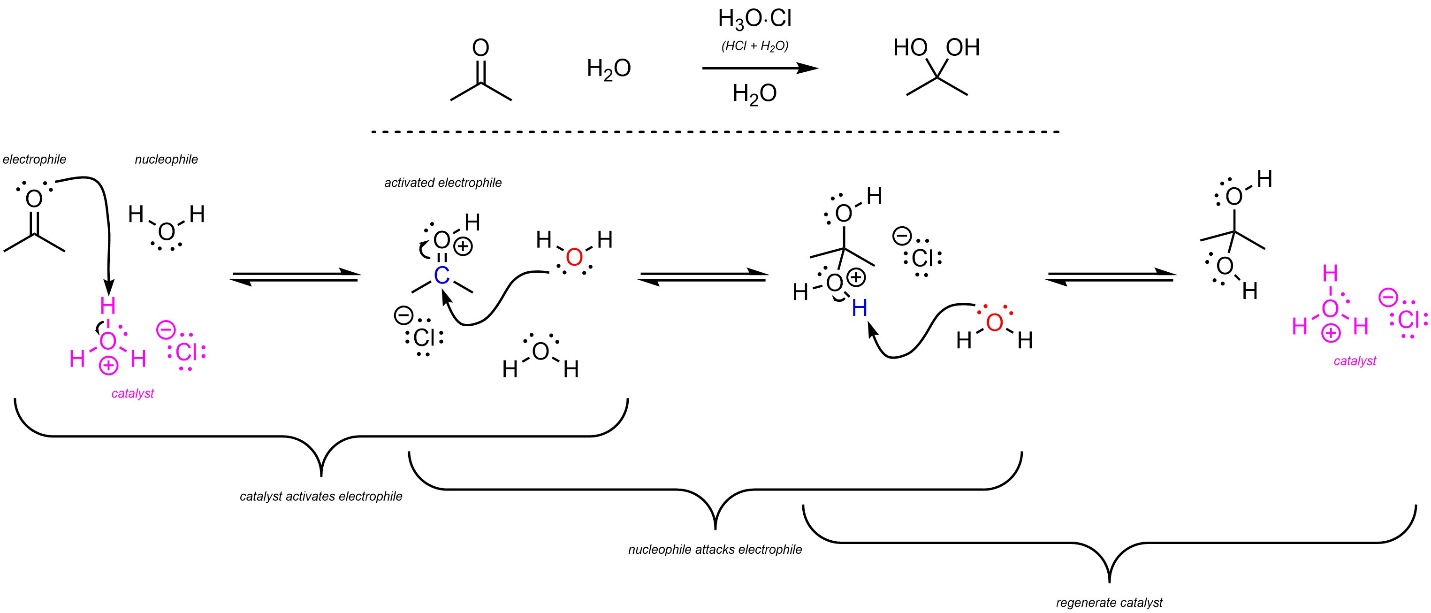
Scheme 7.8 – Reaction Mechanism for Water Attacking Acetone with Acid Catalysis.
7.5.3. Additional Examples – Catalysis for Hemiacetal and Acetal Formation
If an alcohol is used in place of water, a hemiacetal is formed (Scheme 7.9; Et = ethyl = CH2CH3). Either base or acid catalysis is effective. The mechanisms for these reactions are identical to those for acid/base catalyzed hydrate formation.
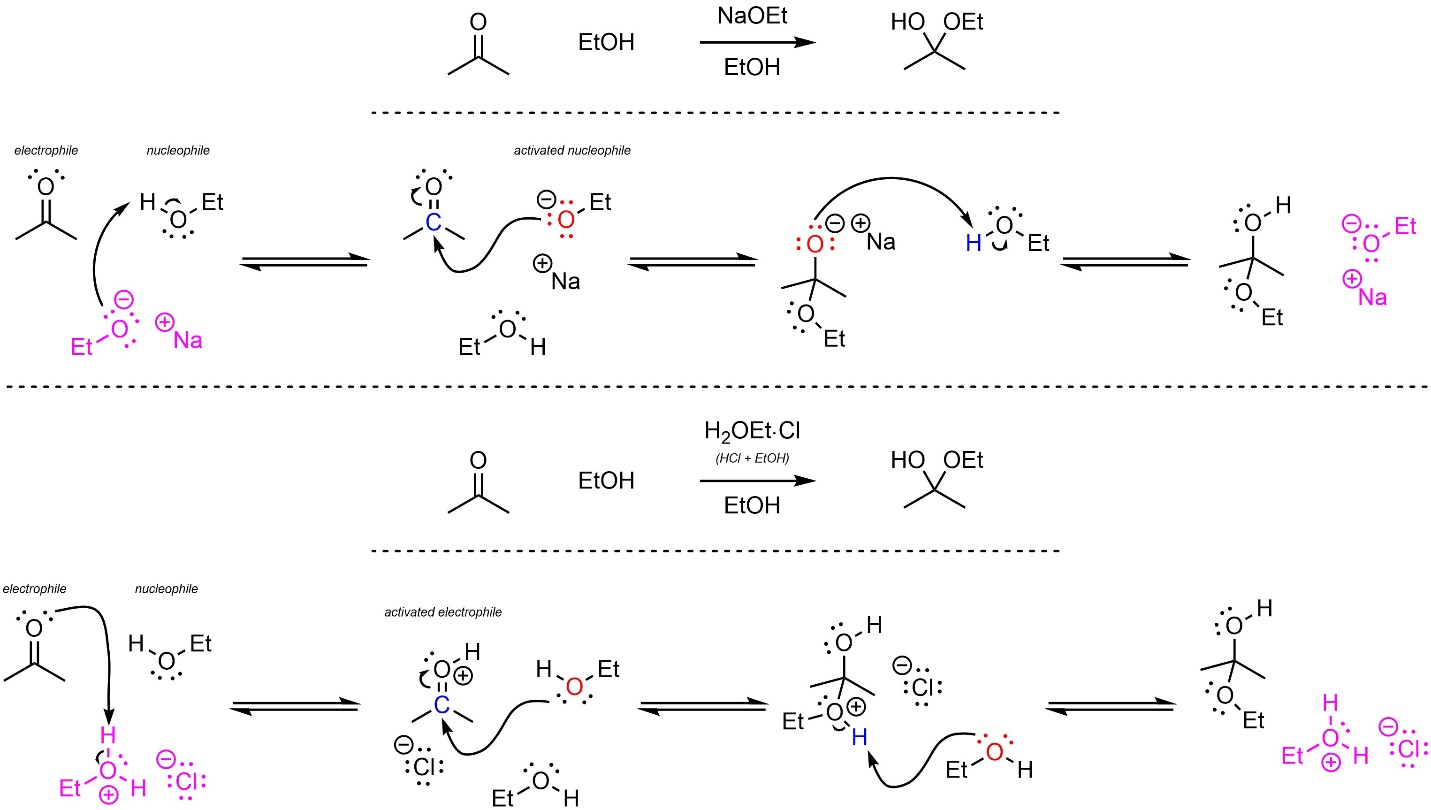
Scheme 7.9 – Reaction Mechanisms for Ethanol Attacking Acetone with Base and Acid Catalysis.
If base catalysis is used, the reaction ends with the formation of the hemiacetal. However, if acid catalysis is used (and there is more than one equivalent of alcohol in the reaction) then the hemiacetal undergoes another reaction (Scheme 7.10). This leads to the formation of an acetal. A common way to accomplish this is by using the alcohol as the solvent, providing a (very) large excess
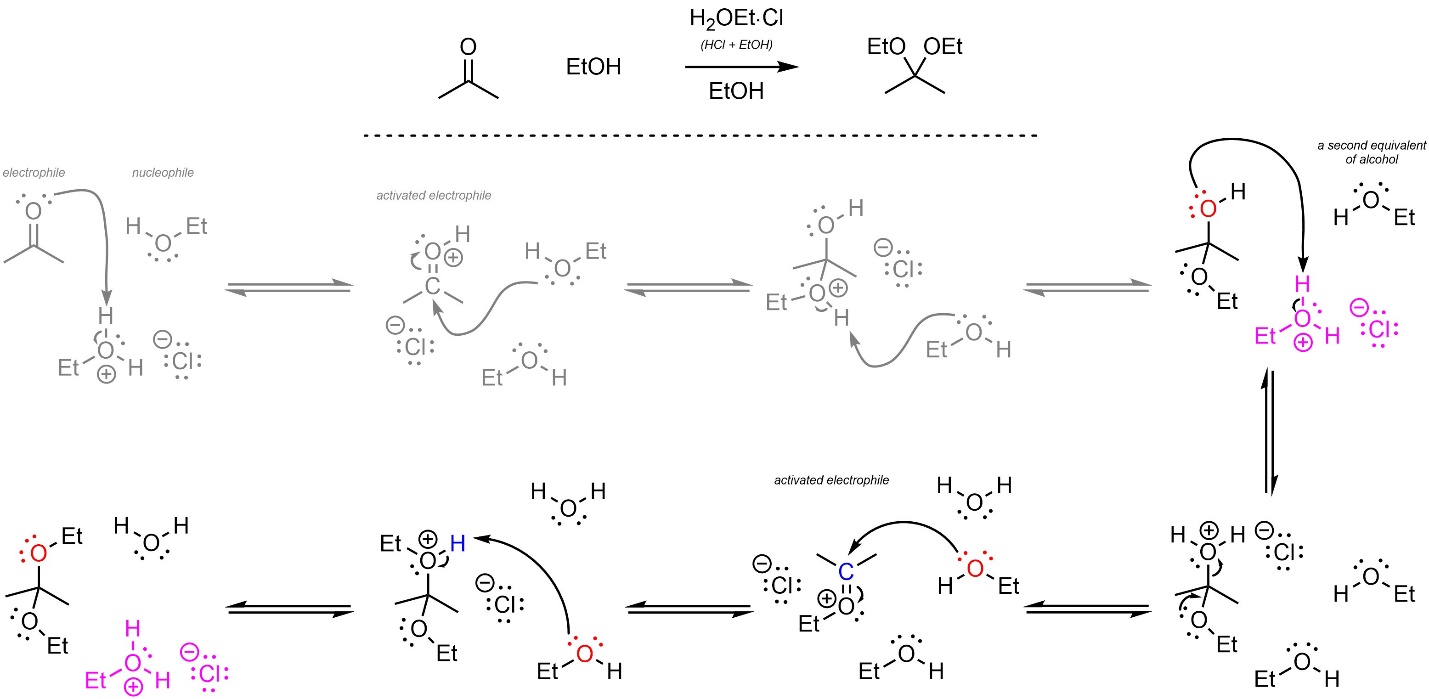
Scheme 7.10 – Reaction Mechanisms for Ethanol Attacking Acetone with Acid and Base Catalysis.
Acetal formation highlights an important consideration of catalysis (Scheme 7.11). Alcohols are weak to moderate nucleophiles, but hemiacetals are very poor electrophiles. Base catalysis does not work for this reaction for multiple reasons, but the most important is that improving the nucleophile will have a negligible effect because the nucleophile is not the problem. Acid catalysis changes the mechanism so that the electrophile becomes an oxonium, converting a very poor electrophile into a very good electrophile.
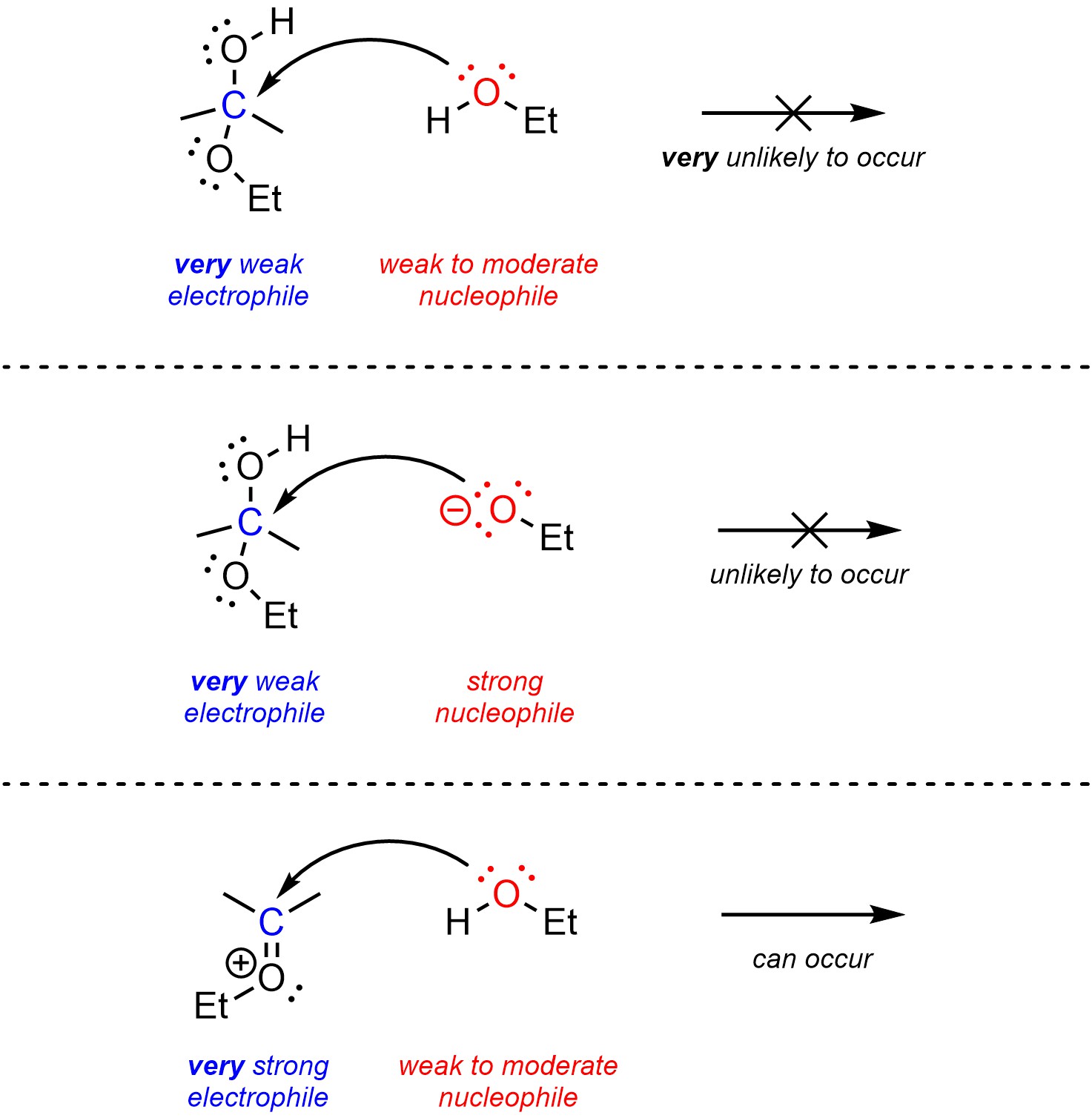
Scheme 7.11 – Reaction Mechanisms for Ethanol Attacking Acetone with Acid and Base Catalysis.

