7.4. Oxidation States
Oxidation/Reduction reactions, commonly referred to as Redox reactions, are extensively studied in general chemistry. In the same way that some inorganic reactions may be classed as oxidations or reductions, some organic reactions also fall into these categories. The most common mnemonic for recalling the difference between the two processes is “LEO GER”: Loss of Electrons is Oxidation, Gain of Electrons is Reduction.
In organic chemistry, oxidation and reduction are more commonly associated with the loss or gain of hydrogens (Figure 7.10). For example, the conversion of a carbonyl to an alcohol is considered a reduction reaction, while the reverse would be considered an oxidation reaction.
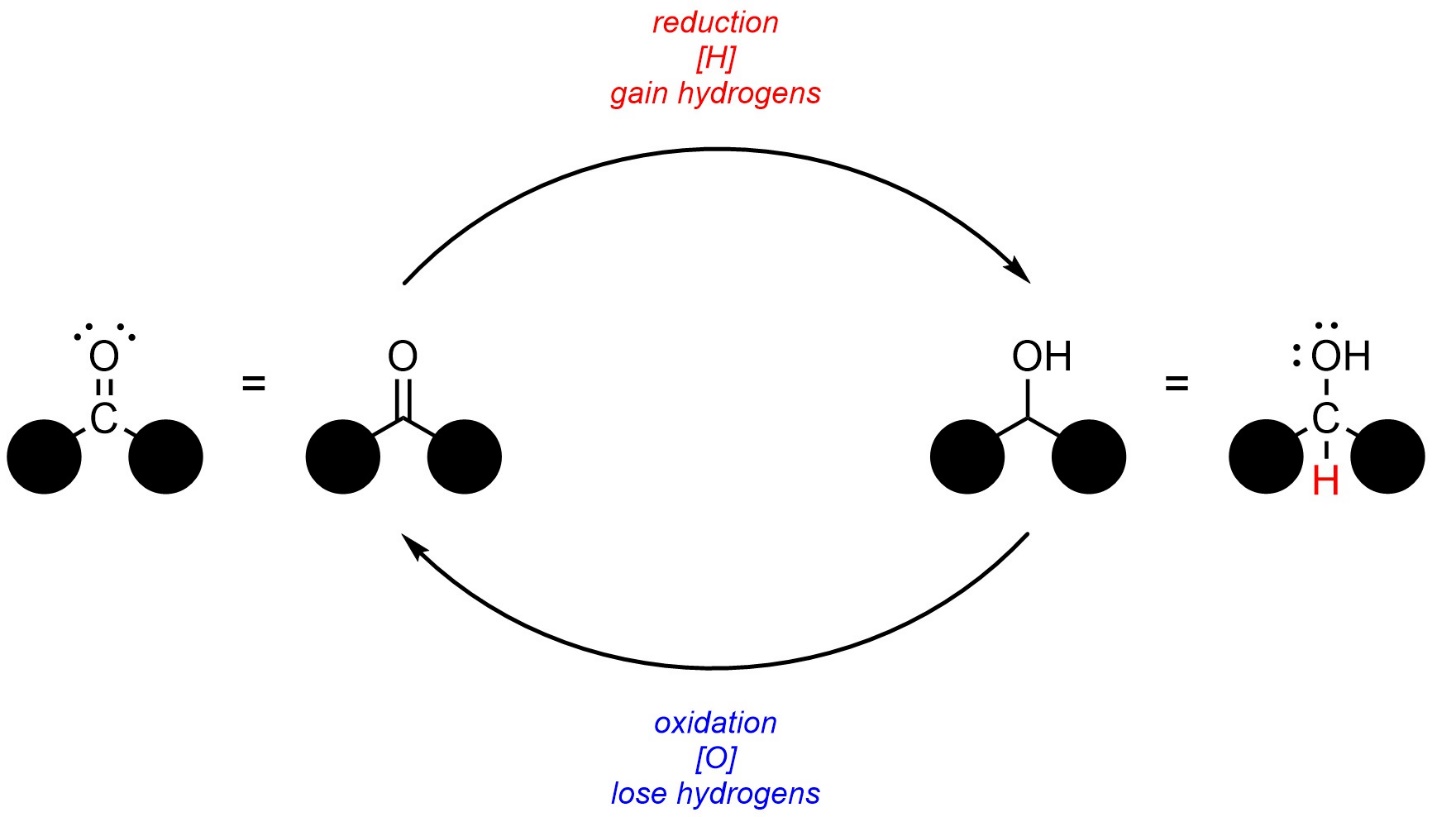
Figure 7.10 – Redox Cycle of a Carbonyl and Alcohol.
It is important to remember that oxygen atoms are not required for different oxidation states. The only consideration in organic chemistry is the number of bonded hydrogen atoms (Figure 7.11).
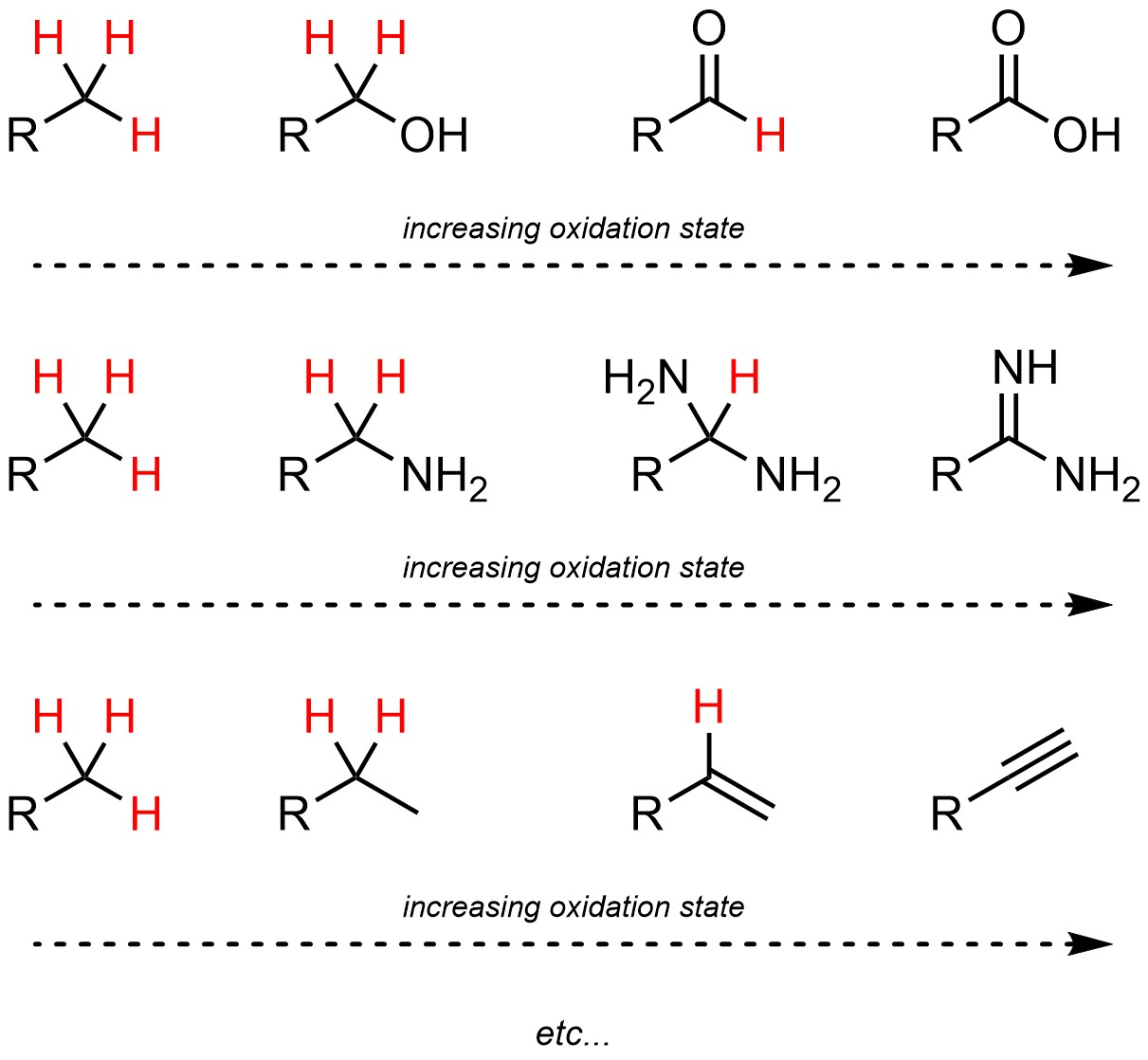
Figure 7.11 – Examples of Comparisons of Oxidation States of Carbon Atoms.

