6.3. Qualitative Estimates of Acidity
It is often useful to compare the relative acidity of two acids. This skill can be valuable for analyzing acid-base reactions but is essential with other types of reactions as well (see Sections 7.2 and 7.7). Almost all acid-base reactions are in equilibrium, with the acid and its conjugate base being on the two sides of the reaction arrow. Anything that shifts equilibrium away from the acid increases relative acidity.
The best way to predict the relative strength of a neutral acid is by looking at the stability of its conjugate base. The more stable the conjugate base is, the more acidic the parent acid is because it is “easier” to give up the proton and become the conjugate base. Any factor that stabilizes an acid’s conjugate base, especially factors that stabilize the negative formal charge, increases acidity.
6.3.1. Electronegativity
Atoms that are more electronegative are better able to accept additional electron density. As a result, they are more stable being anionic. When comparing two acids, with other factors being equal, the acid with the more electronegative atom becoming anionic will be more acidic (Scheme 6.3).
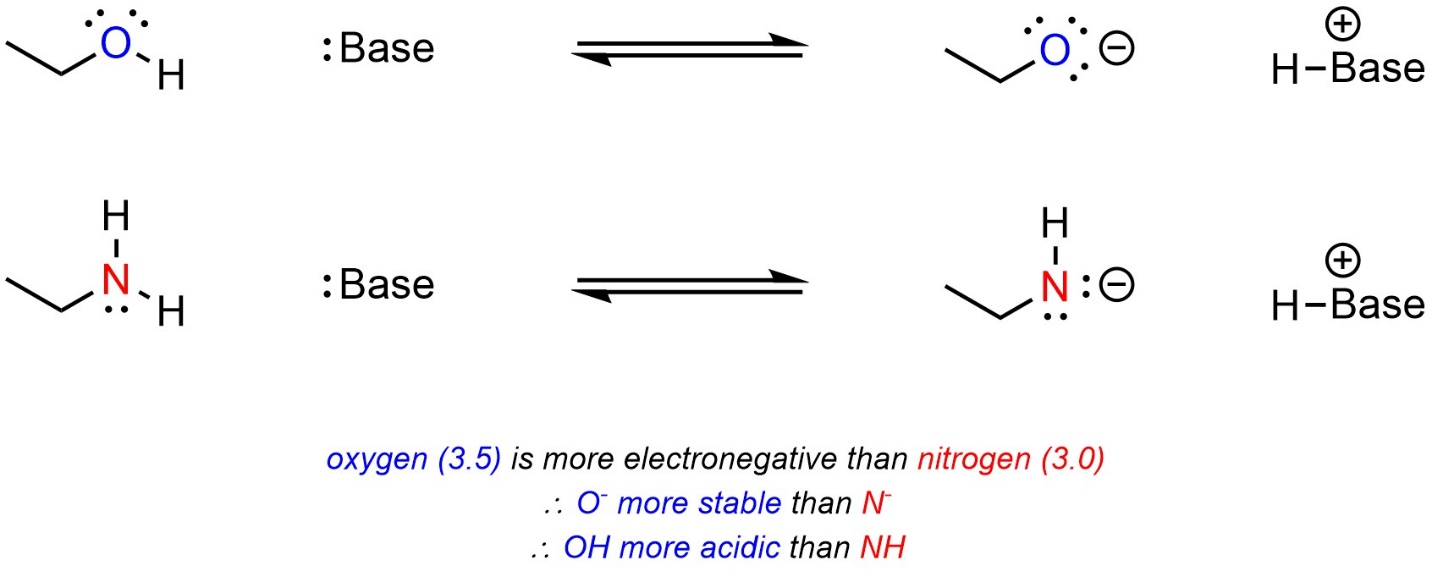
Scheme 6.3 – Example of the Effect of Electronegativity on Relative Acidity.
6.3.2. Induction (Electronegativity II)
Atoms that are more electronegative are better able to accept additional electron density. As a result, they pull electron density away from other areas of the molecule. This is sometimes called induction. When comparing two acids, with other factors being equal, the acid with more electronegative atoms near the site becoming anionic will be more acidic (Scheme 6.4).
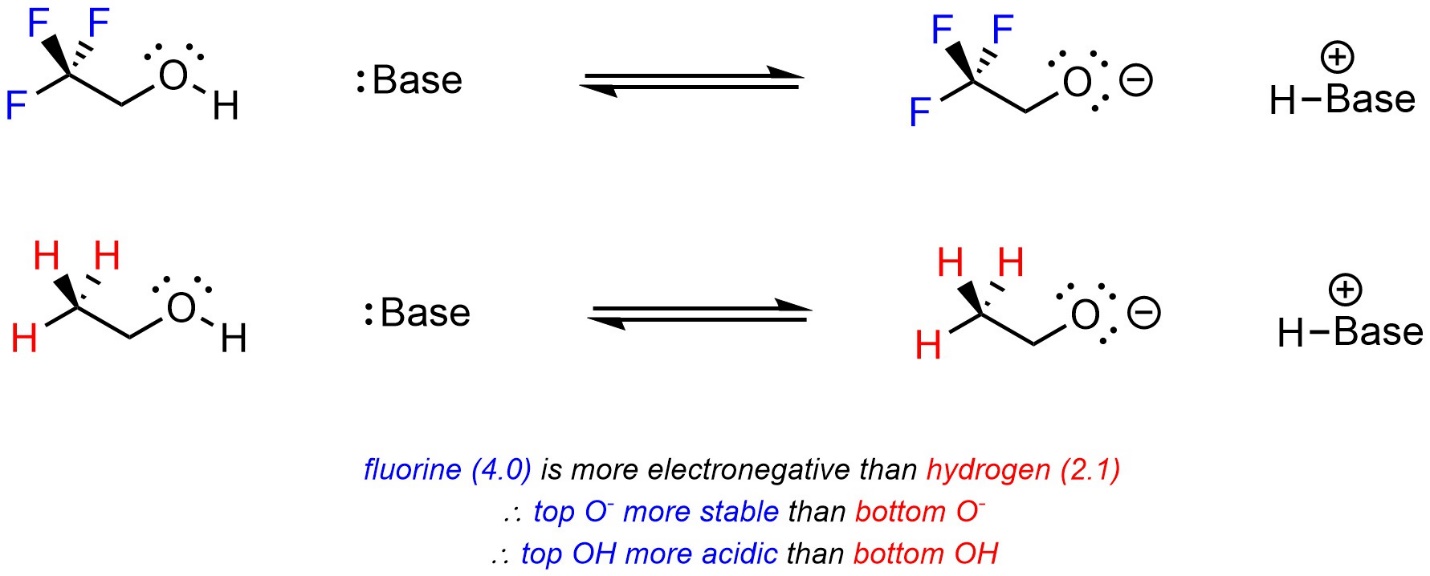
Scheme 6.4 – Example of the Effect of Induction on Relative Acidity.
This results from there being less electron density localized at the specific anionic atom, with it being “drawn” towards the electronegative atoms (Figure 6.4). Contrast the size of the high electron density area (red area) in the two anions.
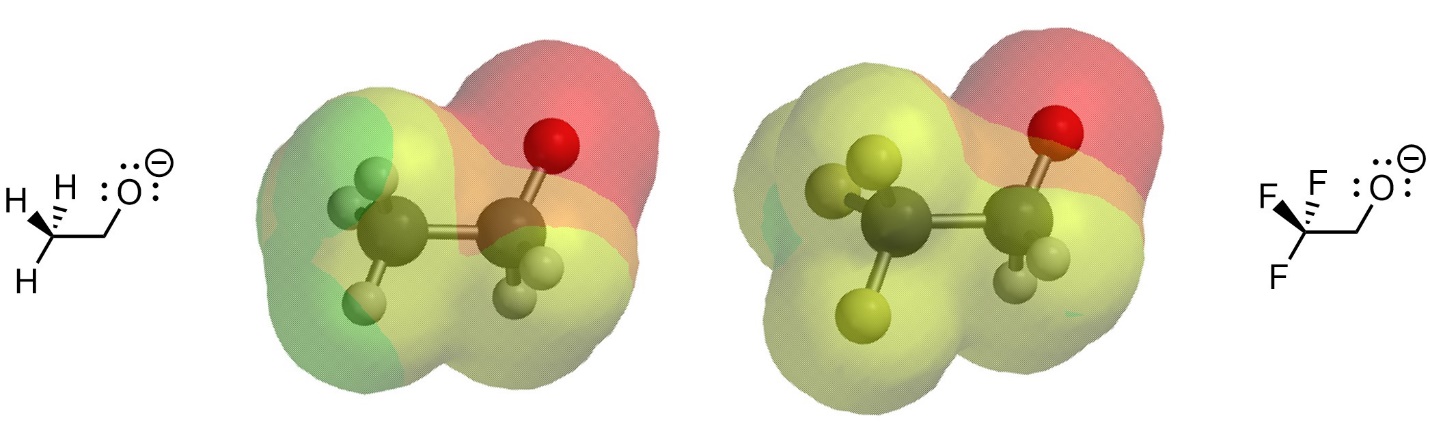
Figure 6.4 – Electron Density in Two Conjugate Bases with Differing Induction.
Strength of induction is dependent on the electronegativity of the atoms involved, how many atoms are involved, and how close they are to the anionic site (Scheme 6.5). At an introductory level, comparisons that require calculations, such as having two fluorines versus having three chlorines, would not be expected.
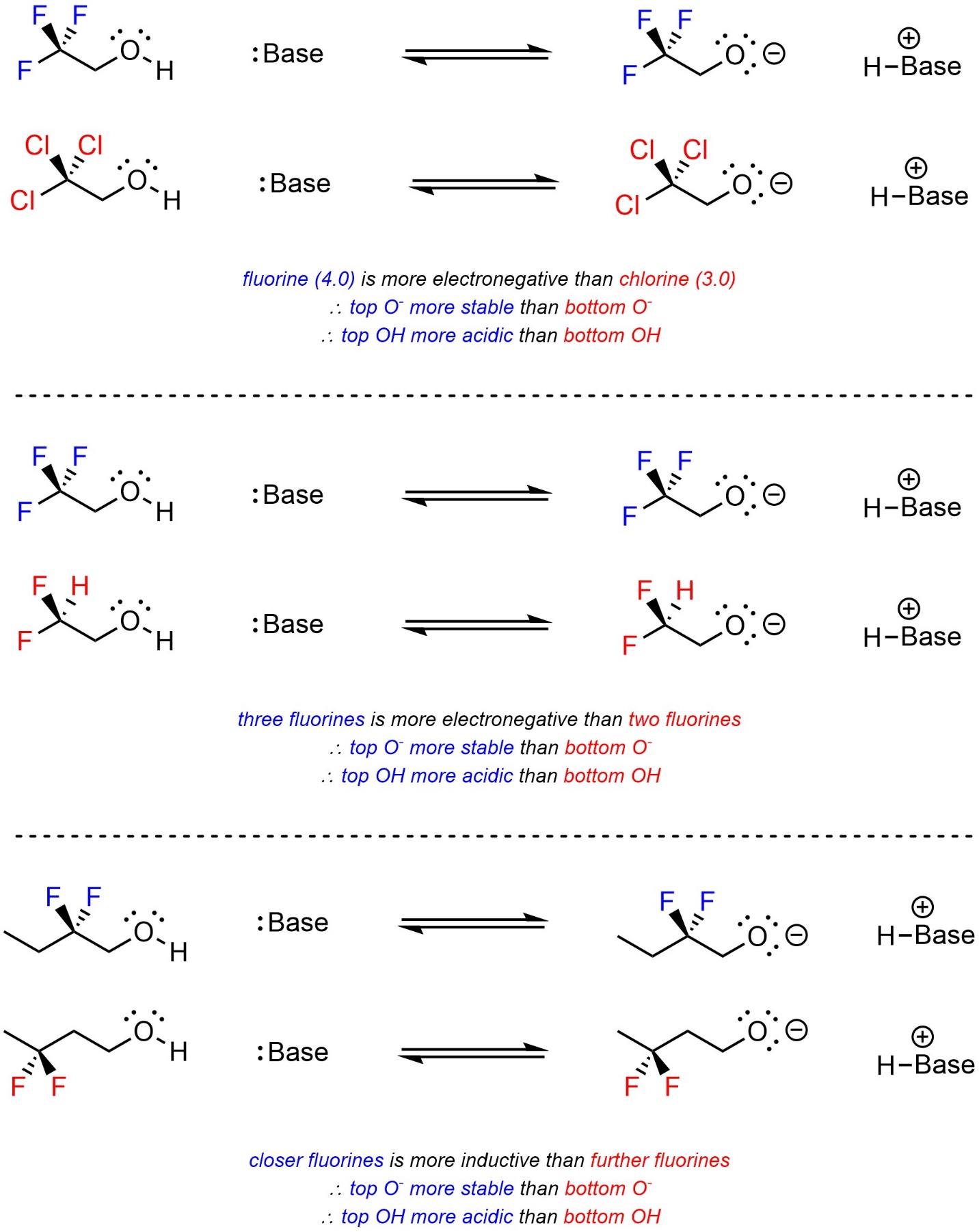
Scheme 6.5 – Examples of Factors Affecting Induction.
6.3.3. Atomic Size
Atoms that are (significantly) larger have access to larger orbitals. For example, the valence orbitals of sulfur are the 3s and 3p orbitals, while the valence orbitals of oxygen are the smaller 2s and 2p orbitals. Having larger orbitals means that electron density is distributed over a larger area, making them better able to accept additional electron density. When comparing two acids, with other factors being equal, the acid with the larger atom becoming anionic will be more acidic (Scheme 6.6). This only applies when the size of the orbitals involved changes (the row in the periodic table); oxygen is larger (with respect to mass) than nitrogen but the valence orbitals in each are the same.
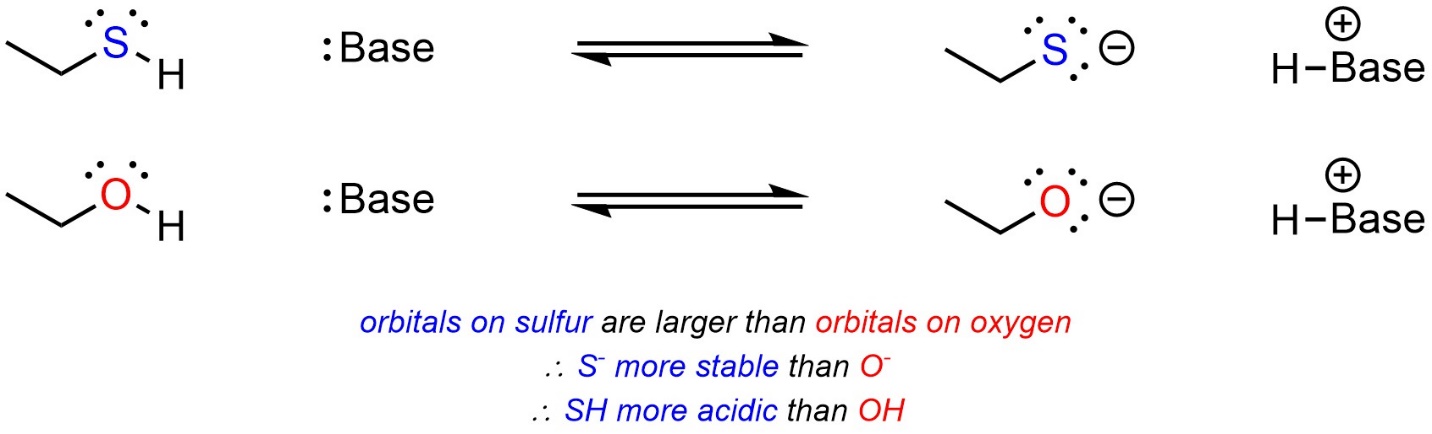
Scheme 6.6 – Example of the Effect of Atomic Size on Relative Acidity.
Sulfur is (significantly) less electronegative than oxygen; the size of atomic orbitals available is a stronger factor than electronegativity.
6.3.4. Hybridization
Atoms with more “s character” in their hybridization state are more stable when carrying a formal charge. Practically, this means that protons bonded to sp hybridized atoms are more acidic than those bonded to sp2 hybridized atoms, which are in turn more acidic than those bonded to sp3 hybridized atoms. This results from several related/competing factors, and an in-depth discussion is best left for more advanced texts. When comparing two acids, with other factors being equal, the acid with more “s character” at the atom carrying the acidic proton will be more acidic (Scheme 6.7).
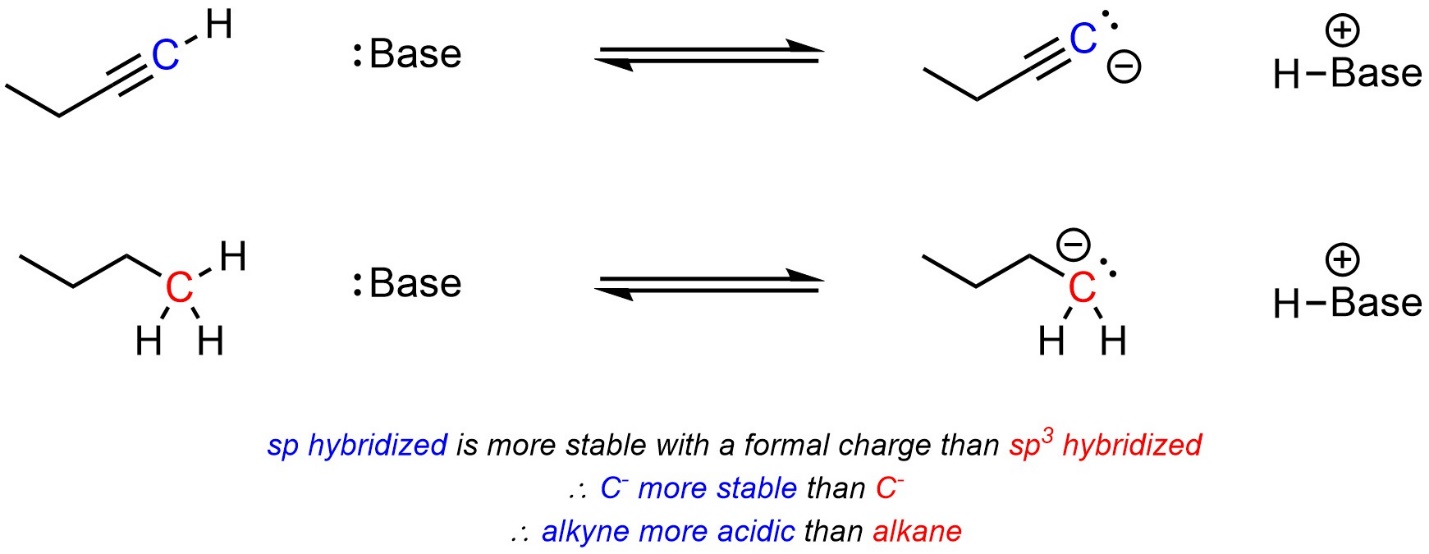
Scheme 6.7 – Example of the Effect of Delocalization on Relative Acidity.
6.3.5. Having Resonance Forms
Recall that having multiple resonance forms provides a large amount of stabilization. When comparing two acids, with other factors being equal, the acid with more delocalization of the anion (more resonance forms) will be more acidic (Scheme 6.8).
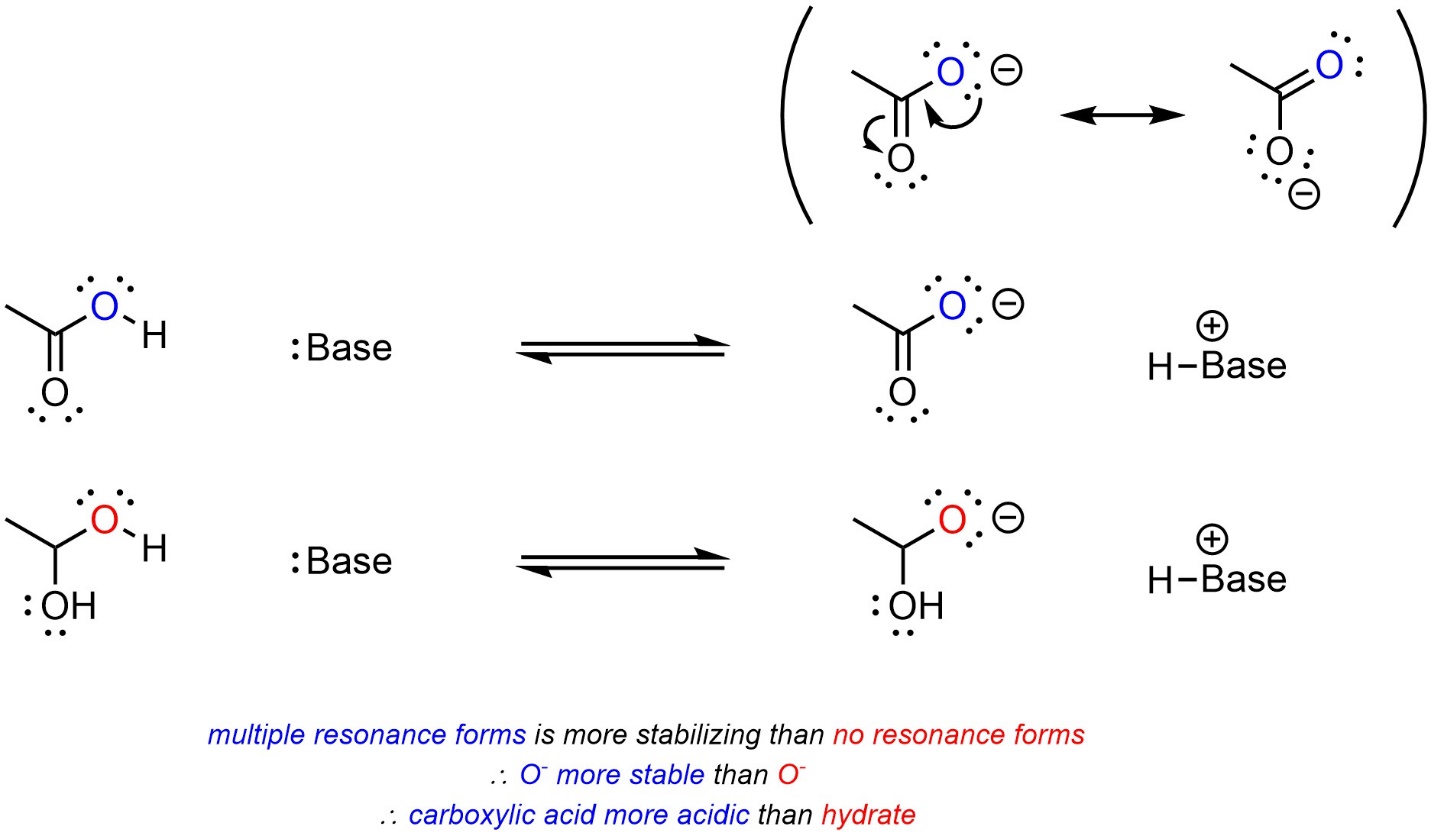
Scheme 6.8 – Example of the Effect of Delocalization on Relative Acidity.
Having resonance forms (delocalization) is, by a wide margin, the most important factor for stability of the conjugate base.
6.3.6. How to Apply These Factors to Cationic Acids
Not all acids are neutral. When comparing neutral acids, the best way to predict the relative strength is by looking at the stability of the anionic form (the conjugate base). The best way to predict the relative strength of a cationic acid is by looking at the stability of its cationic form (the acid itself). The less stable the cation is, the more acidic the acid is because it “wants” to give up the proton and become neutral. Any factor that destabilizes the acid, especially factors that destabilize the positive formal charge, increases acidity. In both cases the relative stability of the species with the formal charge is what is considered.
Electronegativity works the same way as before. Atoms that are more electronegative want more electron density, which means they are less stable being cationic. The acid with the more electronegative atom becoming neutral will be more acidic (Scheme 6.9). Induction from nearby electronegative atoms increases the effective cationic charge by drawing away electron density, which makes the cation less stable. The acid with more induction near the site becoming neutral will be more acidic.
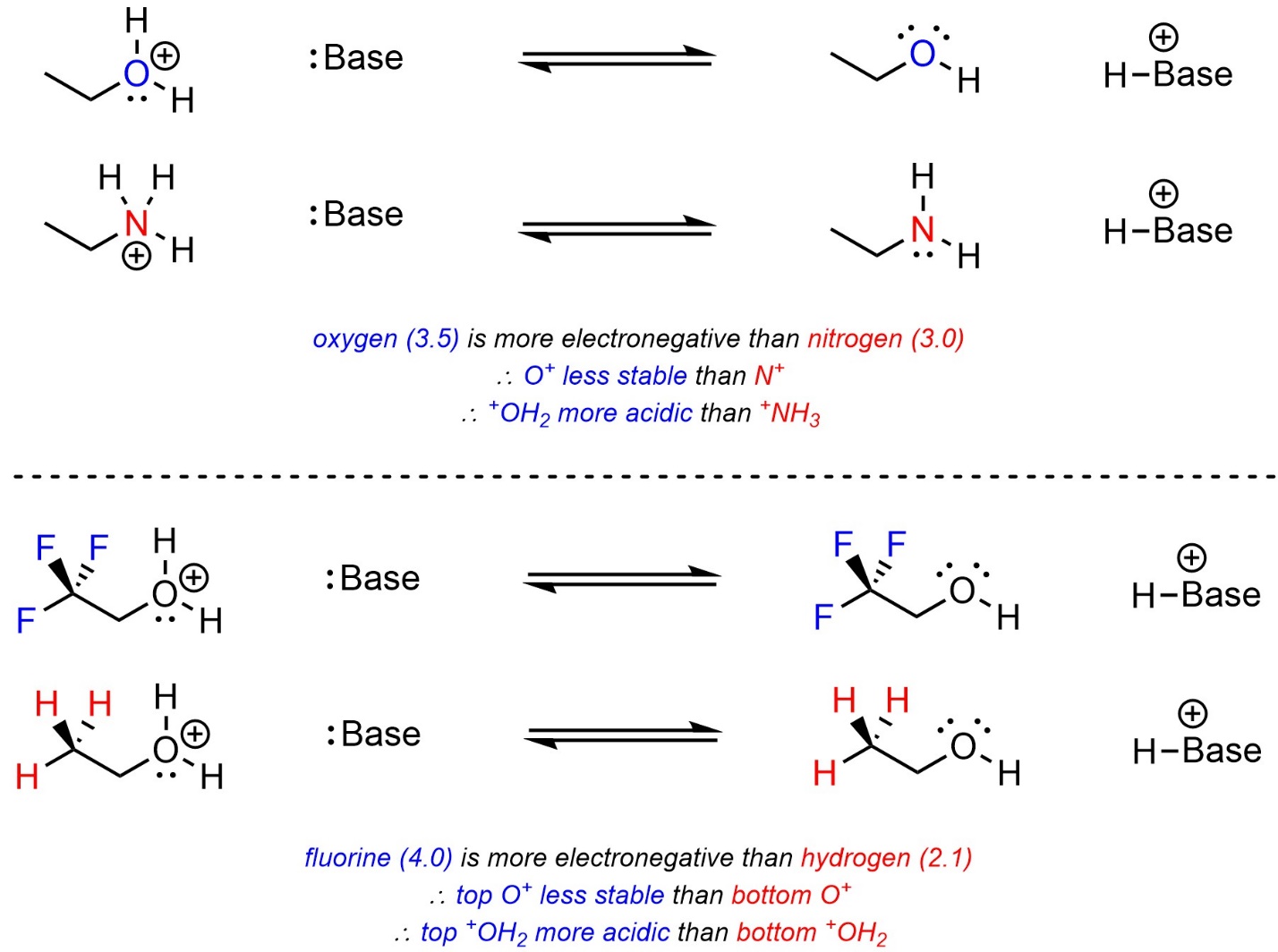
Scheme 6.9 – Examples of the Effects of Electronegativity and Induction on Relative Acidity of Cationic Acids.
Hybridization works in the same fashion as before. However, the effects of atomic size on cationic acid strength are not intuitive and difficult to explain at an introductory level. This text will not consider examples where atomic size plays a role in the relative acidity of cationic acids.
Resonance has more complex effects on cationic acids than on neutral acids.
When the cationic charge can be delocalized through resonance it becomes stabilized, just as the anionic charge in the conjugate base of a neutral acid is. However, since this stabilizes the acid, it shifts equilibrium towards the acid and lowers acidity. The acid with more resonance forms delocalizing the cationic charge will be LESS acidic (Scheme 6.10). This comparison is only valid if the hybridization of the acidic site in each acid is the same. For example, a protonated alcohol is (MUCH) less acidic than a protonated ketone, despite the latter having resonance stabilization.
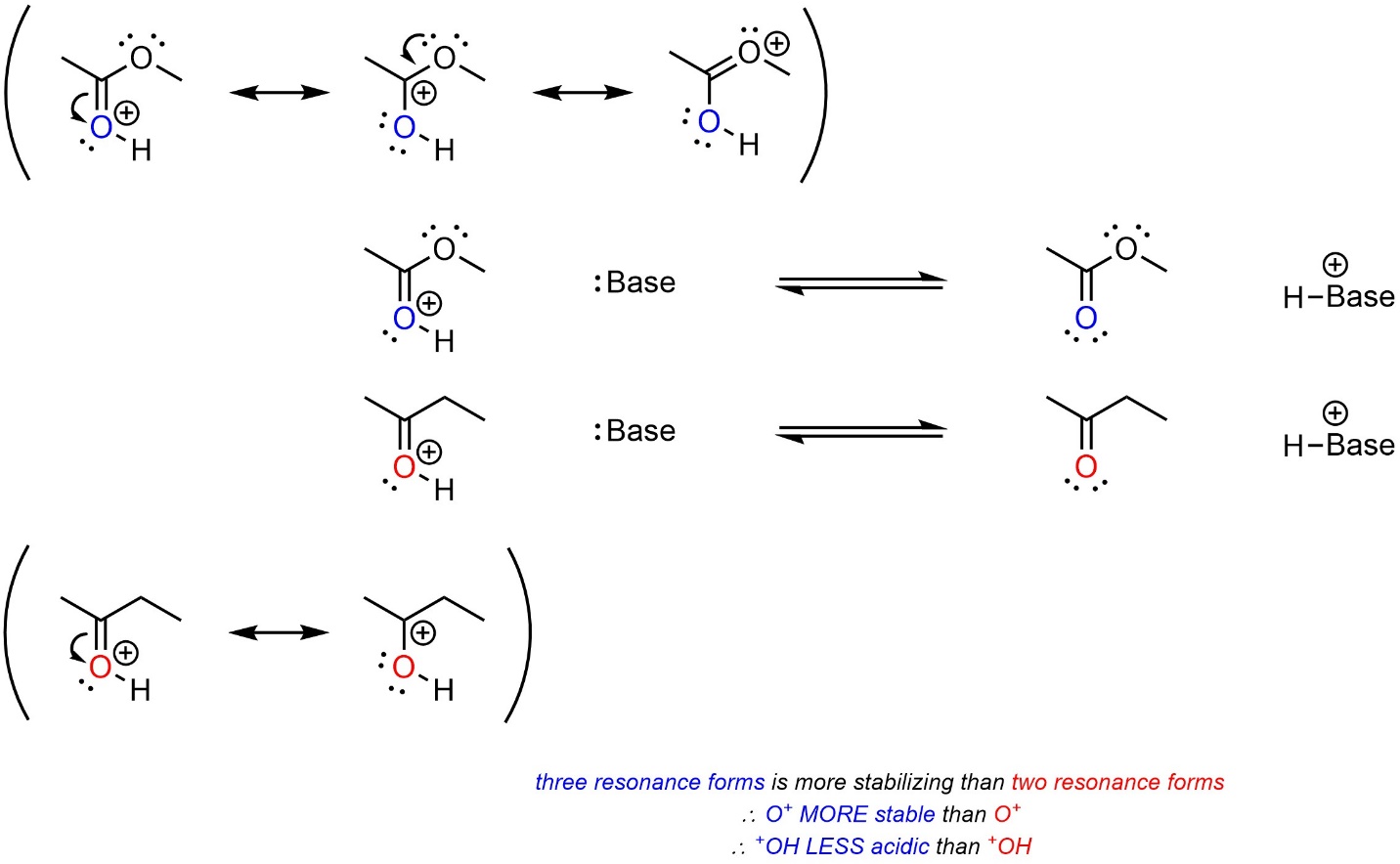
Scheme 6.10 – Example of the Effects of Resonance on Relative Acidity of Cationic Acids with Resonance Stabilization of the Cation.
The effects of resonance on acidity can be substantially more complex. At an introductory level only straightforward analysis is expected.
One factor applies to the stabilization of cations but does not affect anions. Having adjacent alkyl groups stabilizes cationic charges (it has no effect on anionic charges). The more adjacent alkyl groups there are, the more stable the cationic charge becomes. The acid with fewer alkyl groups adjacent to the cationic atom will be more acidic (Scheme 6.11). Why this is true is due to hyperconjugation. However, an in-depth discussion of hyperconjugation is usually considered too advanced for introductory material.
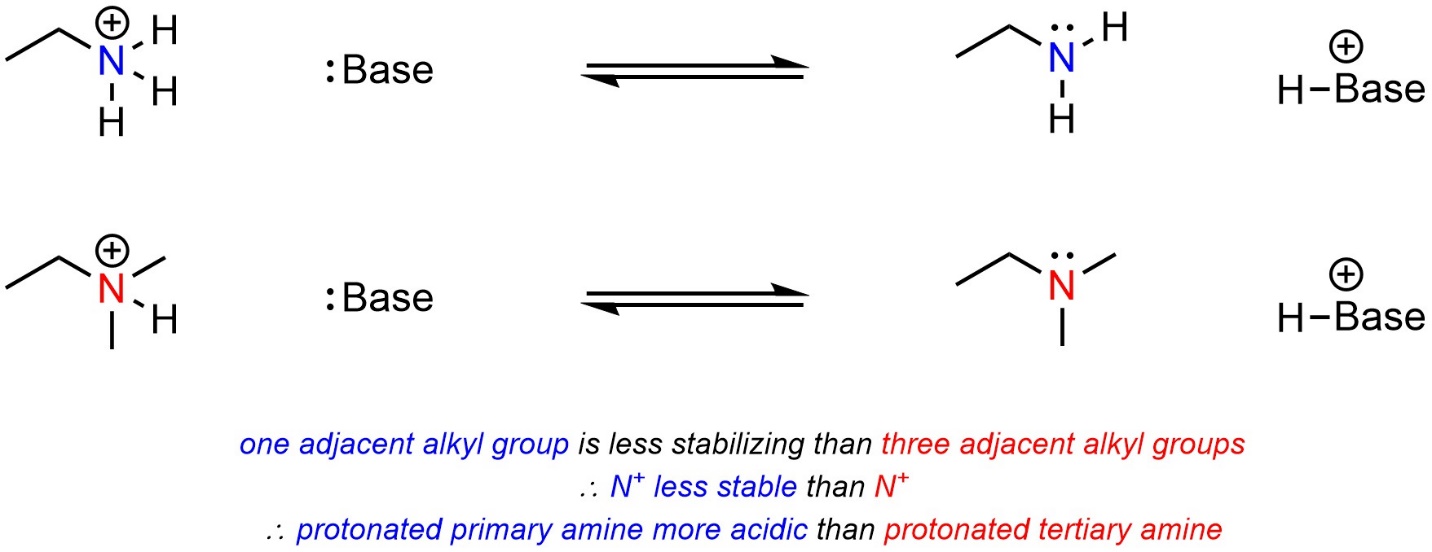
Scheme 6.11 – Example of the Effect of Adjacent Alkyl Groups (Hyperconjugation) on Relative Acidity of Cationic Acids.

