6.2. Reaction Coordinates of Acid-Base Reactions
Acid-base reactions/steps all have a single transition state corresponding to the transfer of the proton from the acid to the base. Generally, strong acids result in reactions that are exergonic, while most weak acids result in endergonic reactions (Figure 6.2).
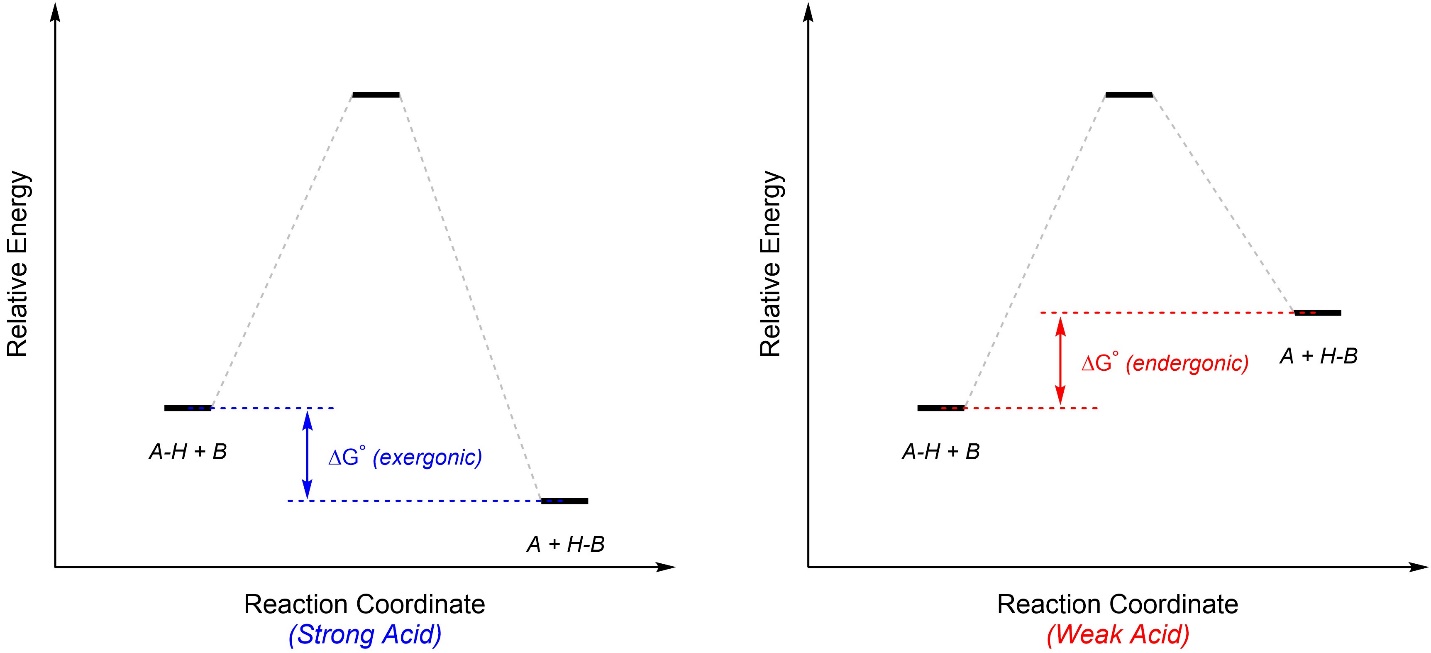
Figure 6.2 – General Reaction Coordinates for Strong and Weak Acids in Acid-Base Reactions.
It is possible to compare the strength of two acids by comparing the relative energies of their products (the conjugate bases). If the starting materials are normalized (placed at the same place on the reaction coordinate) then whichever results in a more stable (lower in energy) product is more acidic (Figure 6.3). Assuming the two reactions are possible, the energies of the two transition states are irrelevant.
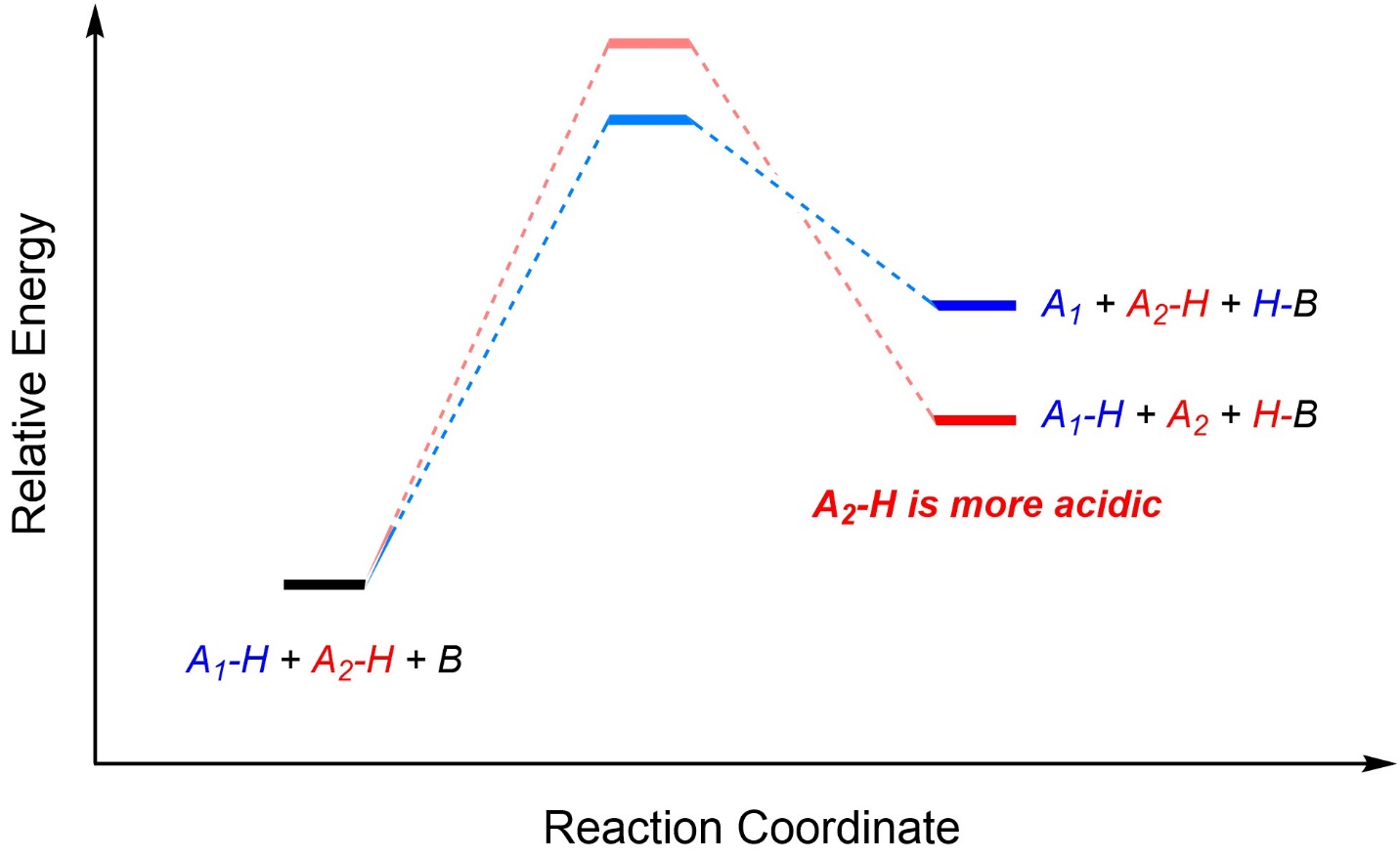
Figure 6.3 – Comparing Acidity Using Reaction Coordinates.
This approach, analyzing the relative stability of the products compared to the starting materials, is the basis for comparing all acids (see Section 6.3).

