5.1. Reaction Equations vs. Reaction Mechanisms
A simple method to show a chemical reaction is a reaction equation. A reaction equation describes what happened, such as when two compounds react with one another to form a third and fourth (Figure 5.1). From the following reaction equation we know that mixing Compound A with Compound B makes Compounds C and D (the exact reaction and compounds are not important at this stage).
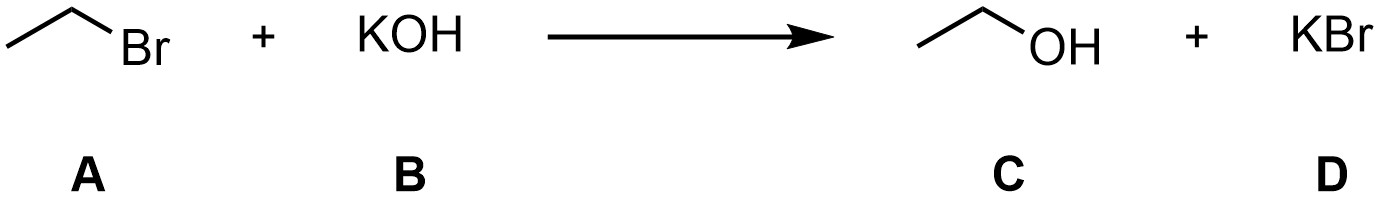
Figure 5.1 – Example of a Reaction Equation.
A more involved method is a reaction mechanism. A reaction mechanism describes how the reaction happened, including how many steps occurred, the order they occurred in, and how the electrons and atoms moved during the reaction (Figure 5.2). Reaction mechanisms are almost always theoretical; it is impossible to “prove” that a specific reaction follows a specific mechanism, but we can collect evidence to support a proposed mechanism and, more importantly, rule out other mechanisms.
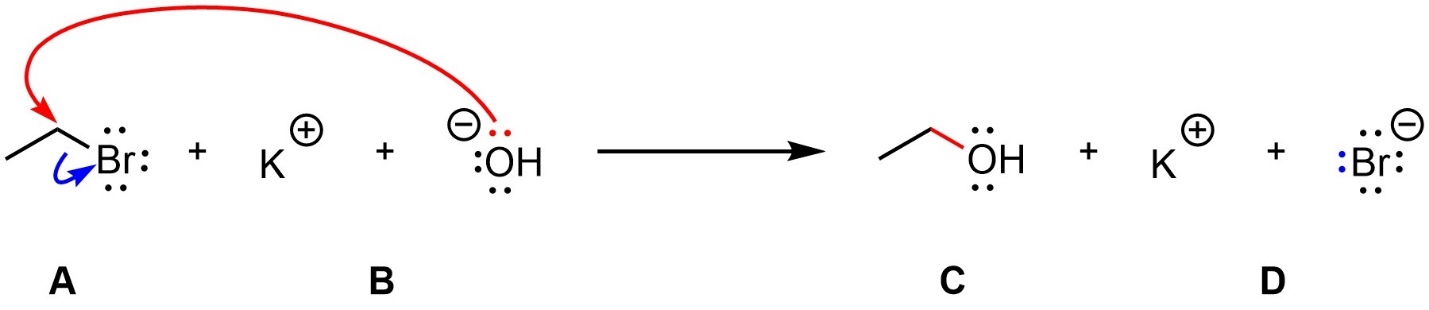
Figure 5.2 – Example of a Reaction Mechanism.
Curved arrows are used to depict electron movement in reaction mechanisms. The exact mechanism above is not important at this stage, but it is useful to see the difference in the amount of information conveyed. For example, with this reaction mechanism we still know that mixing Compound A with Compound B makes Compounds C and D. We also know that a lone pair on oxygen makes a new bond to carbon, that the carbon-bromine bond breaks with those electrons going to bromine, and that these two things occur at the same time. The whole reaction occurs in only one step.

