3.5. Naming Conformations from Rotations Around a σ Bond
The most important types of conformations are frequently discussed and consequently have specific names to refer to them. These conformations correspond to local energy maxima or minima on energy diagrams.
The simplest cases to consider are the conformations around the C-C bond of ethane. Since all of the substituents are the same there are only two important conformations.
When the substituents on the two carbons are directly in front of each other looking down the bond the conformation is eclipsed (i.e. the close atoms are eclipsing the far atoms, like the moon eclipsing the sun; Figure 3.10). This is the highest energy conformation the molecule can adopt because every group in the front is experiencing torsional strain with the corresponding group in the back.
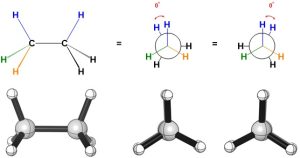
Figure 3.10 – An Eclipsed Conformation of Ethane.
When the substituents on the two carbons are the maximum distance from each other looking down the bond the conformation is staggered (Figure 3.11). This is the lowest energy conformation the molecule can adopt because every group in the front is as far as possible from the corresponding group in the back. Because there are six groups around the C-C σ bond, the maximum dihedral angle is 60° (6 ∙ 60° = 360°), which is the dihedral angle in this case.
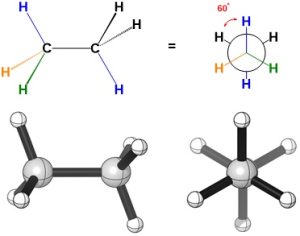
Figure 3.11 – A Staggered Conformation of Ethane.
As the C-C σ bond is rotated the molecule alternates between staggered and eclipsed conformations (Figure 3.12). Since all six groups are identical, the amount of strain experienced in each eclipsed conformation is identical and they have the same relative energy. Notice that the first and last conformations are identical, with the bond having been rotated a full 360°.
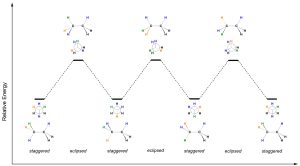
Figure 3.12 – Energy Diagram for the Conformations of Ethane.
Usually chemists are not discussing molecules with only hydrogens as substituents. As a result, there are special names for important conformations describing the angle between the two large groups (or, the two groups being discussed). The conformations of 1,2-dichloroethane will be examined (Figure 3.13), but these terms can be applied to conformations along any σ bond.
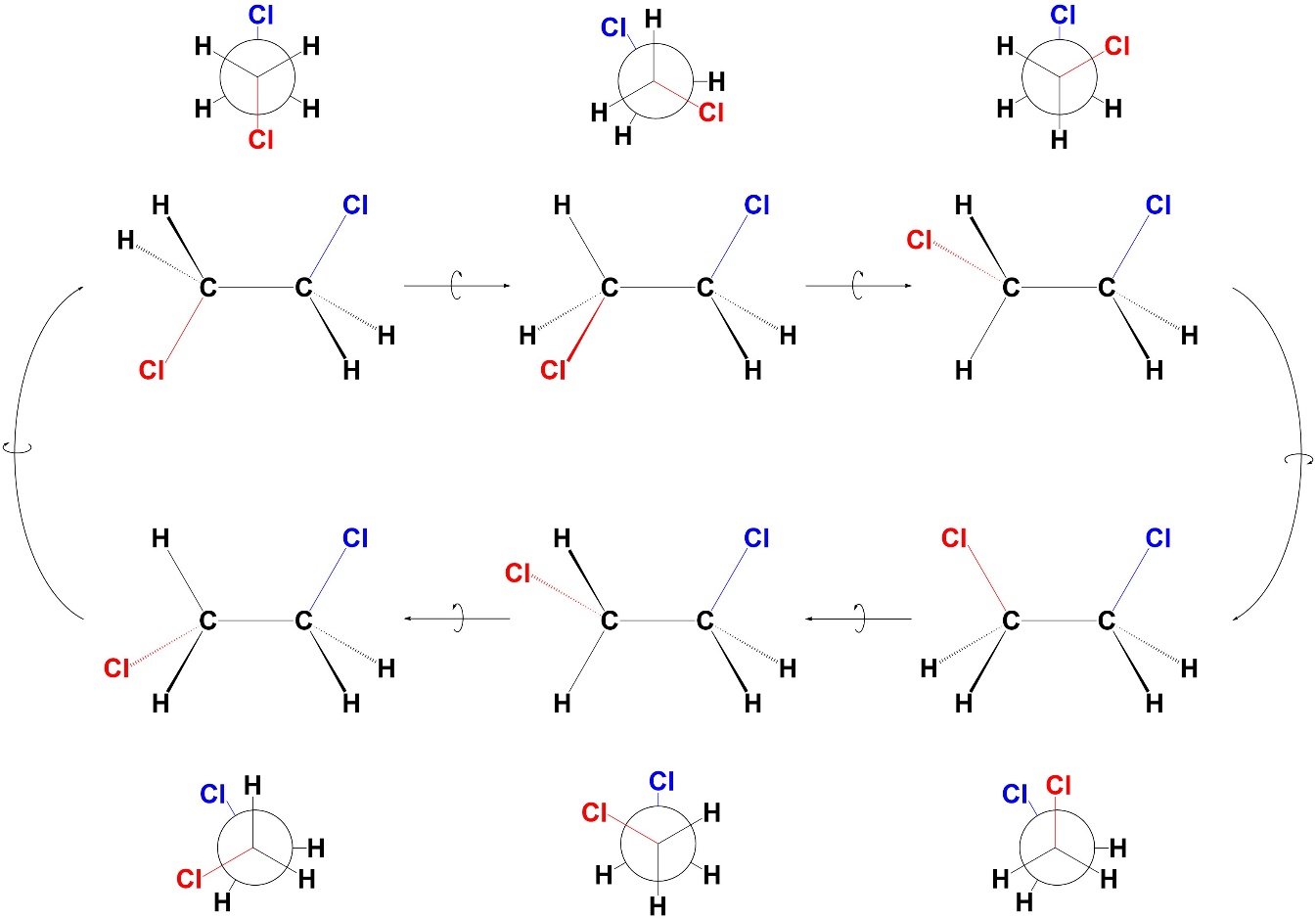
Figure 3.13 – Conformations Resulting from 60° Rotations Around the C-C σ Bond of 1,2-Dichloroethane.
The antiperiplanar conformation (sometimes abbreviated to anti conformation) is the staggered conformation where the two large groups are as far apart as possible (Figure 3.14). They point in opposite directions (“anti” ~ “opposite”) and occupy the same plane (periplanar). The dihedral angle between the two groups is 180°. There is no torsional strain and no steric strain. As a result, this is the most stable conformation.
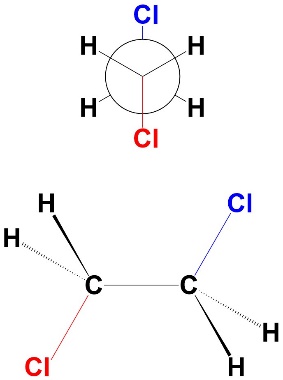
Figure 3.14 – Antiperiplanar Conformation of 1,2-Dichloroethane.
The gauche conformations are the staggered conformations where the two large groups are staggered close to each other (Figure 3.15). The name comes from French, where gauche means “left” (one group can be described as being “to the left” of the other in the Newman Projection). The dihedral angle between the two groups is 60°. There is a very small amount of steric strain (from the two chlorines being somewhat close together) and no torsional strain. As a result, these are the second most stable conformations.
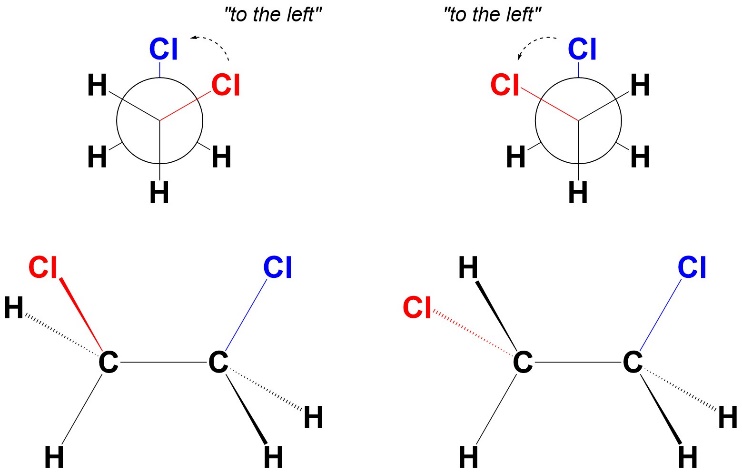
Figure 3.15 – Gauche Conformations of 1,2-Dichloroethane.
Not all eclipsed conformations are the same. The “partially eclipsed” conformations are the eclipsed conformations where the two large groups are eclipsing the small groups (Figure 3.16). They are eclipsed, but not with respect to each other. The dihedral angle between the two groups is 120°. There is torsional strain (each atom experiences torsional strain with the atom it eclipses) and no steric strain. As a result, these are the second least stable conformations.
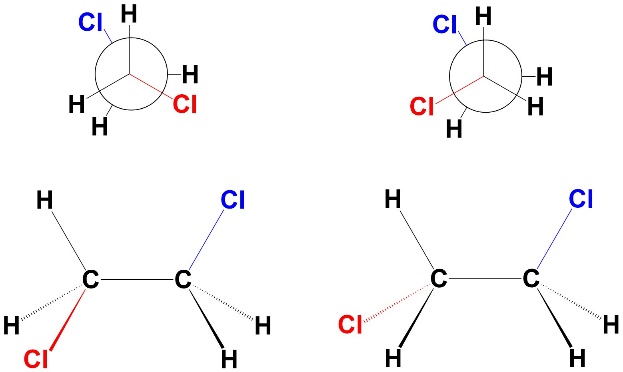
Figure 3.16 – Partially Eclipsed Conformations of 1,2-Dichloroethane.
The “fully eclipsed” conformation is the eclipsed conformation where the two large groups are eclipsing each other (Figure 3.17). Since the large group is in front of the other large group, they experience the maximum amount of eclipsing interactions. The dihedral angle between the two groups is 0°. There is torsional strain (each atom experiences torsional strain with the atom it eclipses) and steric strain (from the chlorines eclipsing each other). As a result, this is the least stable conformation.
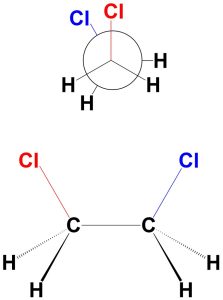
Figure 3.17 – Fully Eclipsed Conformation of 1,2-Dichloroethane.
As the C-C σ bond is rotated the molecule transitions between staggered and eclipsed conformations (Figure 3.18). Since the different conformations experience different types and amounts of strain, they have different energy levels. Notice that the first and last conformations are identical, with the bond having been rotated a full 360°.
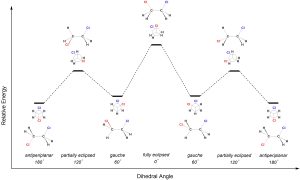
Figure 3.18 – Energy Diagram for the Conformations of 1,2-Dichloroethane.
Often molecules have more than two substituents across a σ bond. If it is obvious which groups are the largest on each side, these names can be used directly. More commonly these names are used as relative descriptors to describe the orientation of two groups with respect to each other (Figure 3.19). Care must be used when doing so. For example, there are two conformations where group X is gauche to group Y, so describing the conformation using only those terms is ambiguous.
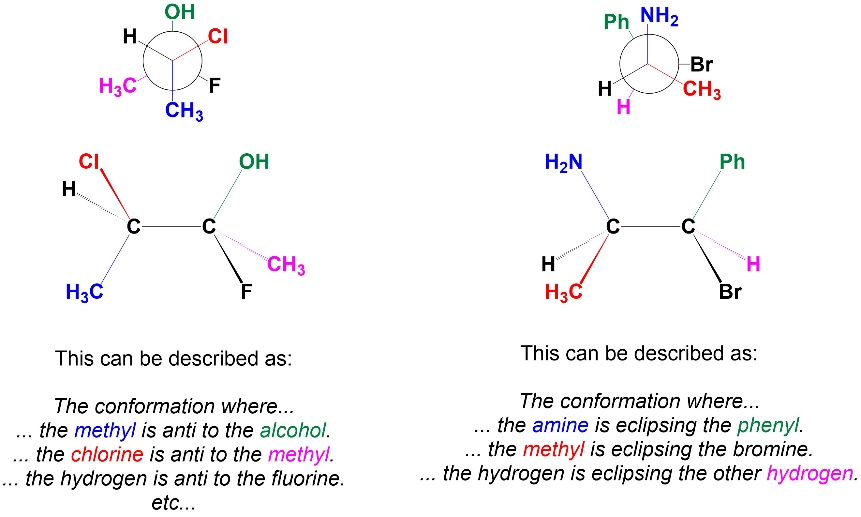
Figure 3.19 – Examples of Conformational Names as Relative Descriptors.

