2.4. Effects of Intermolecular Forces
Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. This means that bulk physical properties such as melting and boiling points are strongly influenced by the types and degrees of intermolecular forces a compound possesses. It also influences how the molecule will interact with other compounds, such as solvents.
2.4.1. Boiling and Melting Point Effects
Boiling and melting points are directly influenced by the amount of intermolecular forces a molecule can exert on another equivalent of itself. The more, and stronger, the interactions it can have with an equivalent of itself, the higher the melting/boiling points will be (i.e. the more energy it takes to disrupt/break those interactions).
Simple alkanes only have dispersion forces. The melting/boiling points are directly affected by how much dispersion can be experienced. As chain length increases, dispersion increases because there is more surface area that can be contacted between molecules (Figure 2.36).
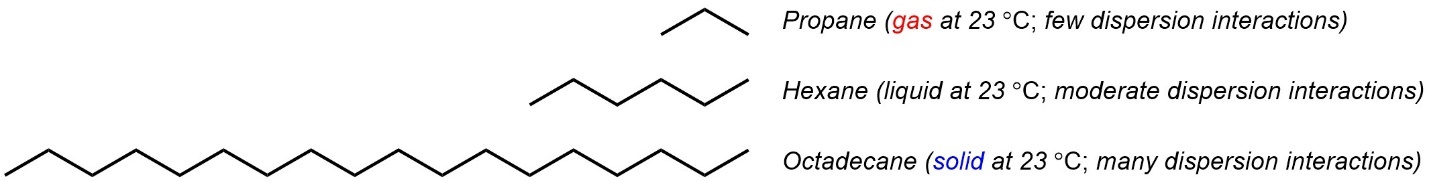
Figure 2.36 – Examples of the Effect of Chain Length on Melting/Boiling Point.
Having branches decreases how close two molecules can get with each other, so it decreases the amount of dispersion interactions (Table 2.1). This makes sense: it is easier to stack 2×4 planks close together than it is to stack tree branches close together.
Table 2.1 – Effects of Branching and Cyclization on Boiling Points of Six-Carbon Alkanes.
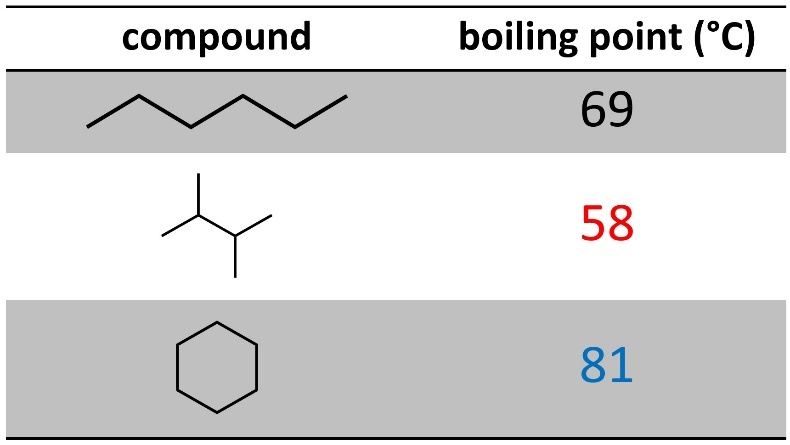
Being cyclic will also affect the amount of dispersion experienced. This is more complicated and has to do with the degrees of freedom (flexibility) the molecule has. Rings are not as flexible because the two ‘ends’ are attached to each other. This means there are fewer ‘shapes’ it can be in (see Section 3.6), which leads to more efficient packing. This increases the amount of dispersion interactions.
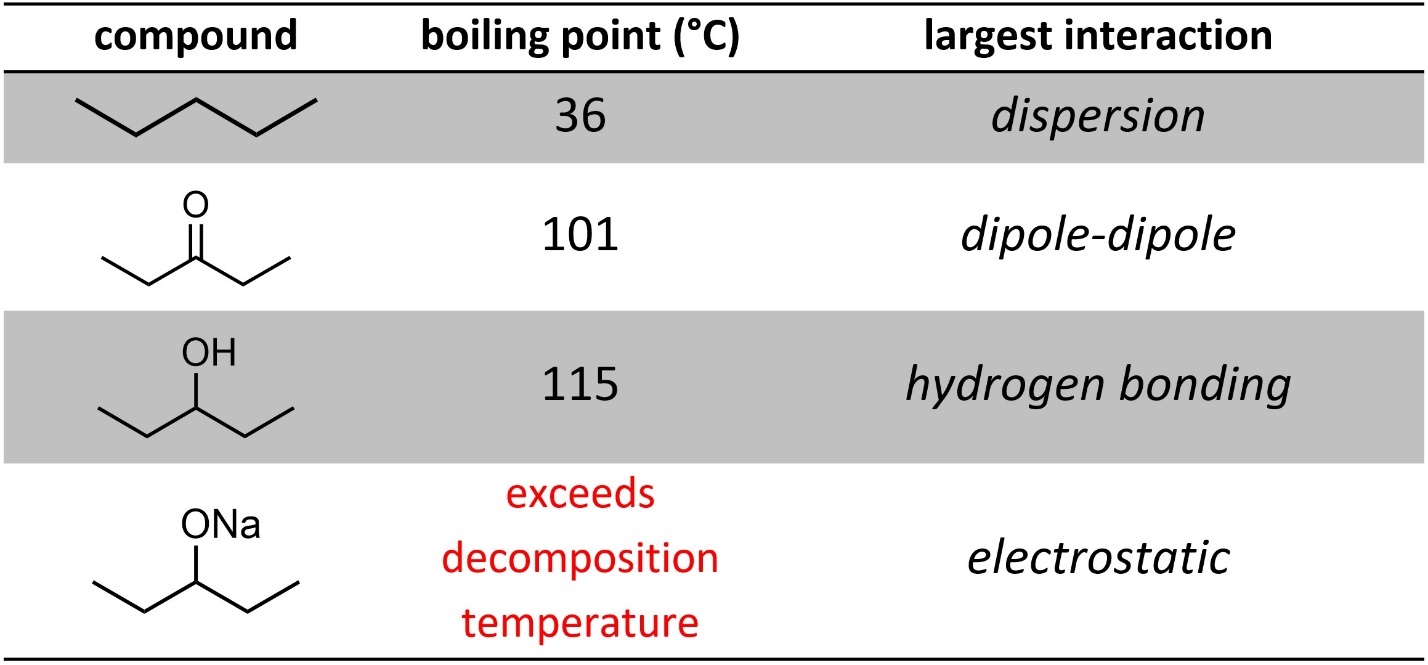
When other forces are present the situation becomes more complex. Generally, dispersion is significantly weaker than dipole-dipole interactions, which are slightly weaker than hydrogen bonding interactions (Table 2.2). By a wide margin electrostatic interactions have the most effect.
Table 2.2 – Examples of Effects of Intermolecular Forces on Boiling Point.
2.4.2. Solubility Effects
The types of interactions available will affect how well a molecule can interact with another bulk compound such as a solvent. In general, having similar interactions to the solvent will allow better compatibility. More commonly this is expressed as like dissolves like. For example: molecules that are polar dissolve (much) better in polar solvents than non-polar solvents; molecules that can accept hydrogen bonds dissolve (much) better in protic solvents than aprotic solvents; etc…
Often dispersion forces will compete with dipole-dipole interactions (including hydrogen bonding). For example, water as a solvent is both polar and capable of hydrogen bonding. Molecules like hexane and octadecane (Figure 2.36 above), which can only interact with other molecules via dispersion forces, are functionally insoluble in water. This carries to many other organic molecules, even polar ones, that are not capable of hydrogen bonding interactions.
2.4.2.1. How to Classify Solvents
Solvents are generally classed using two parameters. First, whether the molecule is Polar or Non-Polar (whether it does or does not have a permanent net dipole). Second, whether the solvent is Protic or Aprotic (whether it can or can not donate a hydrogen bond). The chemical structure of the solvent dictates which category it falls into for each parameter (Figure 2.37).
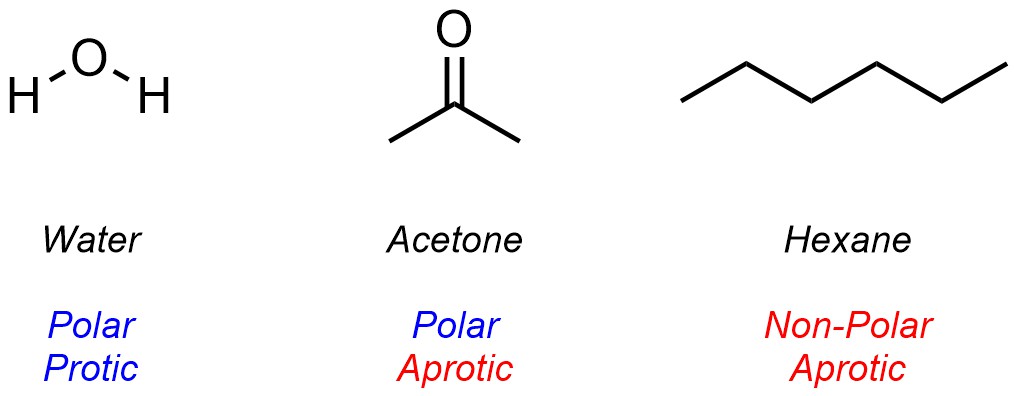
Figure 2.37 – Examples of Common Solvents Classed Using Polar/Non-Polar and Protic/Aprotic Parameters.
These traits can be used to determine whether the solvent is appropriate for solubilizing a given molecule by comparing its interactions with the solvent’s. For example, it is possible to choose the ‘best’ solvent from the set above for a set of molecules (Figure 2.38). Notice that in most cases multiple solvents may work (not all interactions need to align perfectly for solubility to be possible) but the ‘best’ solvent will be one which aligns well with all of a compound’s intermolecular forces.
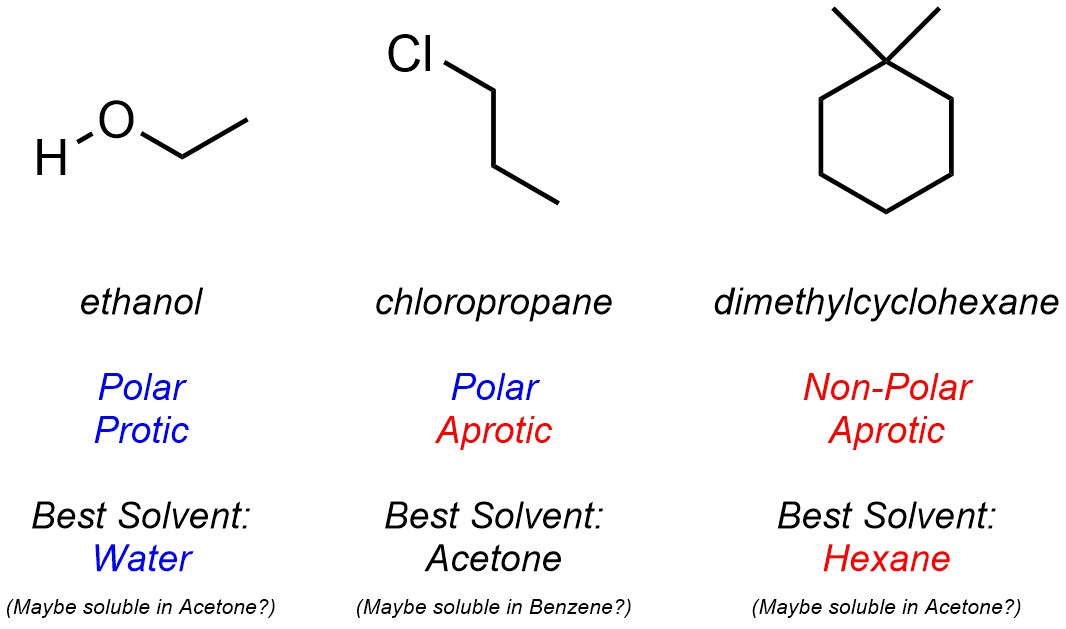
Figure 2.38 – Examples of Matching Solvents with Compounds Based on Intermolecular Forces.
2.4.3. Hydrophilicity and Hydrophobicity
Water is so common that there are special terms to refer to whether compounds (or parts of compounds) like to dissolve in it (hydrophilic; “water loving”) or not (hydrophobic; “water fearing”).
Hydrophobic compounds (or parts of compounds) aggregate and interact with each other rather than water (Figure 2.39). This can cause insolubility or lead to aggregates.
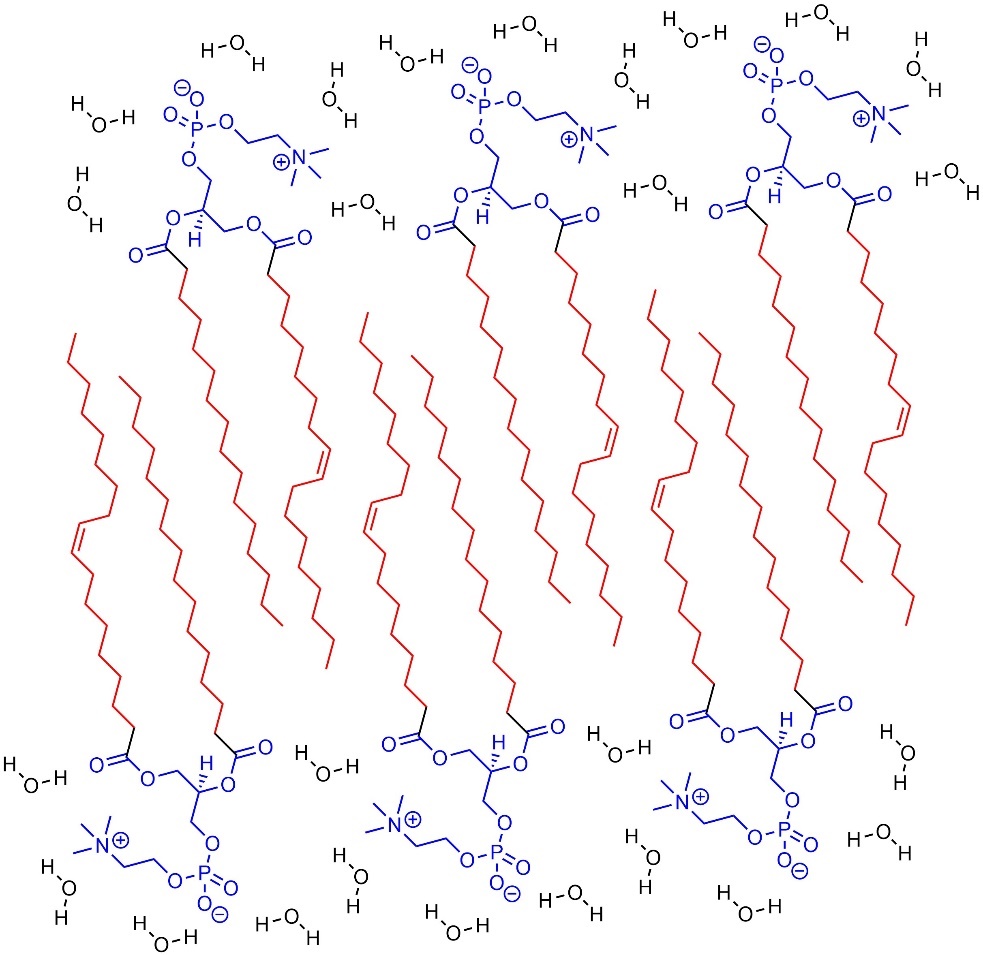
Figure 2.39 – Example of Aggregation from Hydrophobic Areas of Molecules.
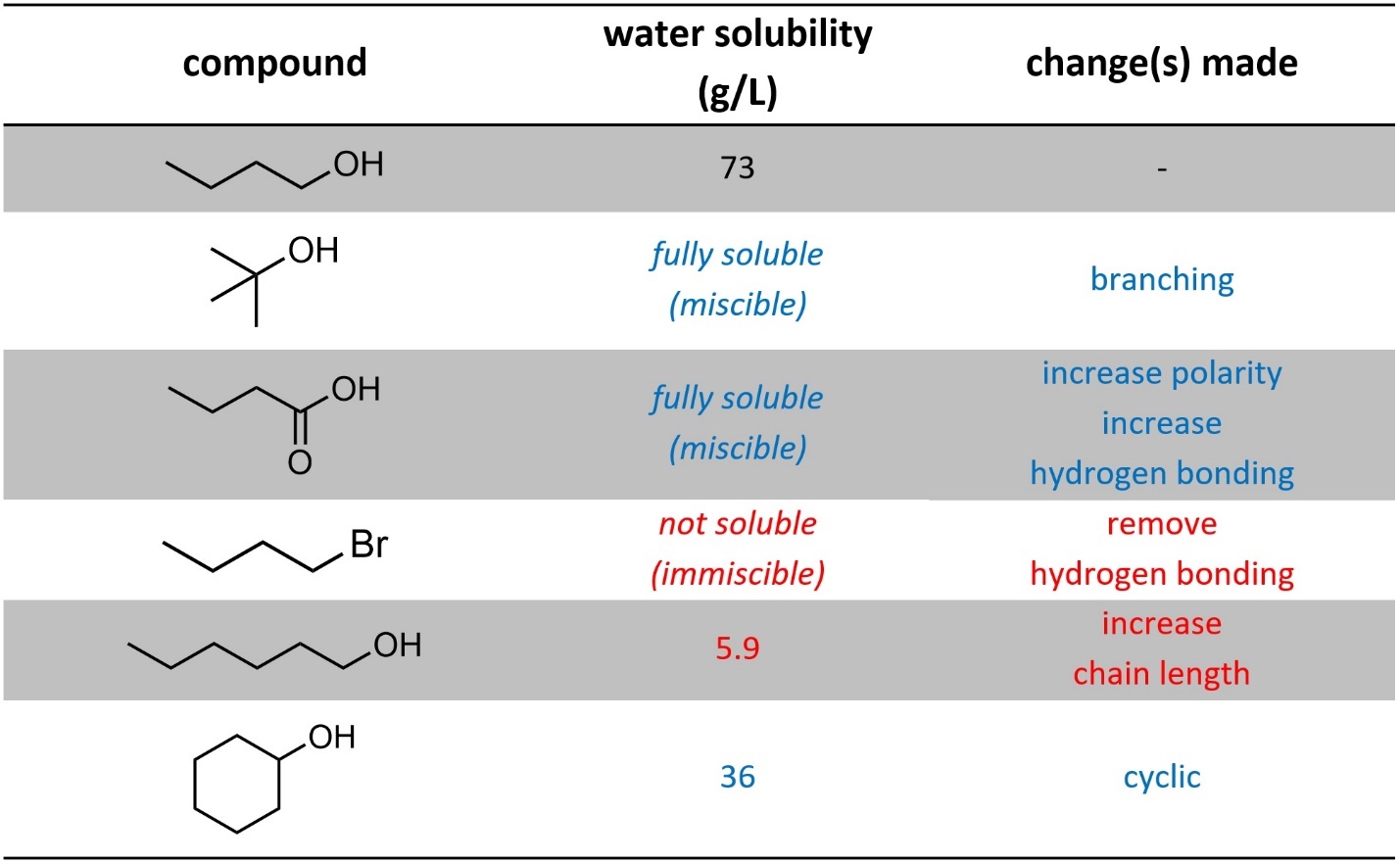
As the ratio of hydrophilic interactions (dipole-dipole, hydrogen bonding, electrostatic) to hydrophobic interactions (dispersion) increases, solubility in water increases. This means that factors that decrease dispersion interactions, such as branching, will increase solubility in water. Factors that increase dispersion, such as longer chains and cyclic systems, will decrease solubility in water (Table 2.3).
Table 2.3 – Effects on Water Solubility from Changes in Intermolecular Forces.
As even polar aprotic organic molecules may be insoluble with water (see Section 2.4.2), generally both dipole-dipole interactions and hydrogen bonding are required to be hydrophilic. Typically salts (compounds with electrostatic interactions) are also highly hydrophilic.

