12.6. Reaction: Elimination of H-X (Dehydrohalogenation)
Two types of elimination reactions occur very frequently in synthesis. Dehydrohalogenation, the elimination of a strong halogen acid (H-X), is a very common elimination reaction (Scheme 12.13). It is possible for this reaction to occur with (Lewis) acid catalysis. However, in the overwhelming majority of cases chemists prefer to simply use a strong base such as sodium hydroxide. Note that although H-X is formally eliminated it will not actually be a product of the reaction; the strong base will form water (or an alcohol) and a salt.
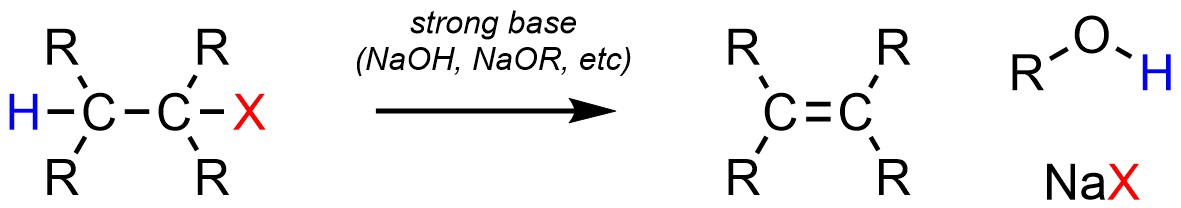
Scheme 12.13 – Generalized Reaction Equation for Elimination of H-X to Form an Alkene.
12.6.1. Mechanism
Depending on the structure of the starting material it is possible for this reaction to proceed through an E2 or E1 mechanism. However, when using a strong base often the E2 mechanism is faster for these reactions. The mechanism for this reaction is the same as a standard E2 elimination (Scheme 12.14; see Scheme 12.2). The strong base (NaOH) removes a proton, creating a new conjugate acid (water). This occurs at the same time that electrons from the old bond form a new π bond to an adjacent carbon by ejecting the leaving group (the halide). Recall that while most halides are excellent leaving groups, fluorine is an exception; this reaction will not work with a fluoride (F–) leaving group.
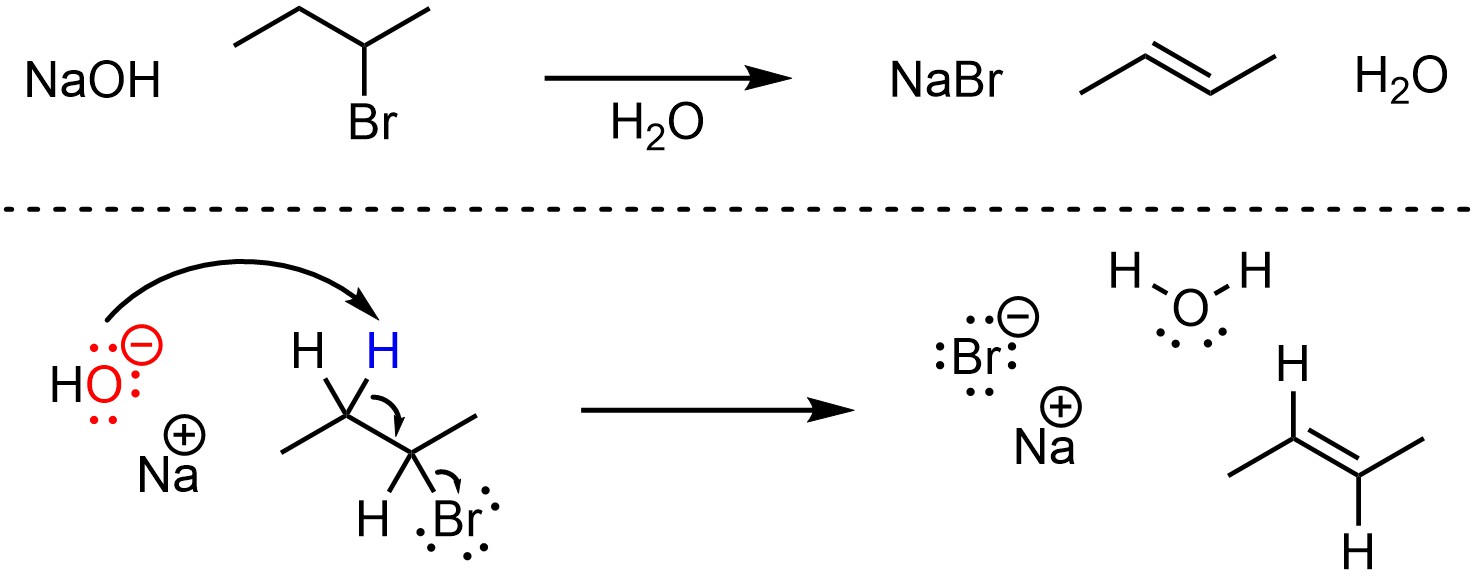
Scheme 12.14 – Reaction Mechanism for E2 Elimination of 2-Bromobutane with Sodium hydroxide.

