12.2. Elimination Reactions: E2 Reactions
Elimination reactions can occur with one or two steps in the mechanism. Reactions that follow the E2 mechanism have one step. The main difference from their counterpart is that they have a very specific geometric requirement to occur. This effect affects regioselectivity.
12.2.1. Mechanism
Elimination reactions that follow the E2 mechanism have one step (Scheme 12.2). The base removes a proton, creating a new conjugate acid. This occurs at the same time that electrons from the old bond form a new π bond to an adjacent carbon by ejecting the leaving group. The new π bond forms at the same time that both old bonds break.
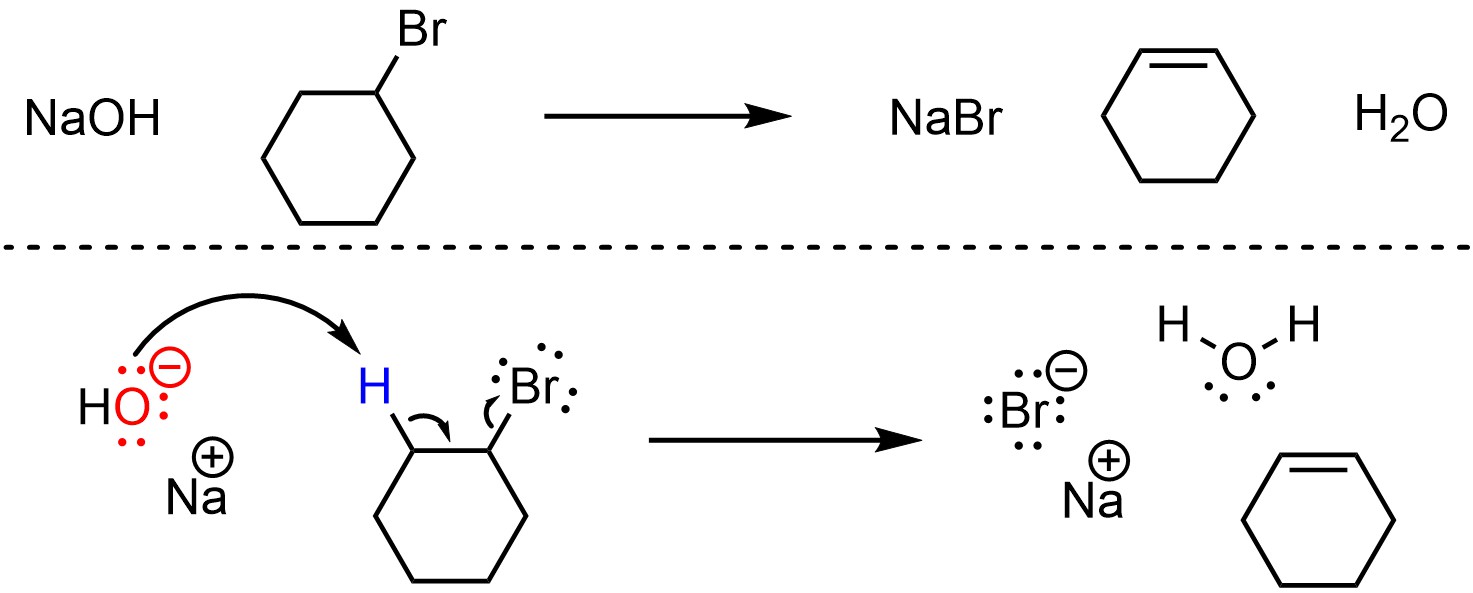
Scheme 12.2 – Reaction Mechanism for E2 Elimination of Bromocyclohexane with Sodium hydroxide.
Depending on the specific reaction that is occurring there may be additional steps before or after the substitution step. For example, the nucleophile/base or leaving group may be activated prior to the substitution, or there may be a protonation or deprotonation after the elimination. These extra steps do not change the classification of the mechanism for the elimination step; if the new π bond forms at the same time the old bonds break, it is still classed as an E2 elimination reaction.
12.2.2. Reaction Coordinate and Rate-Determining Step
There is only one step in an E2 elimination reaction. By definition, it is the rate-determining step (Figure 12.1). The overall reaction is exergonic.
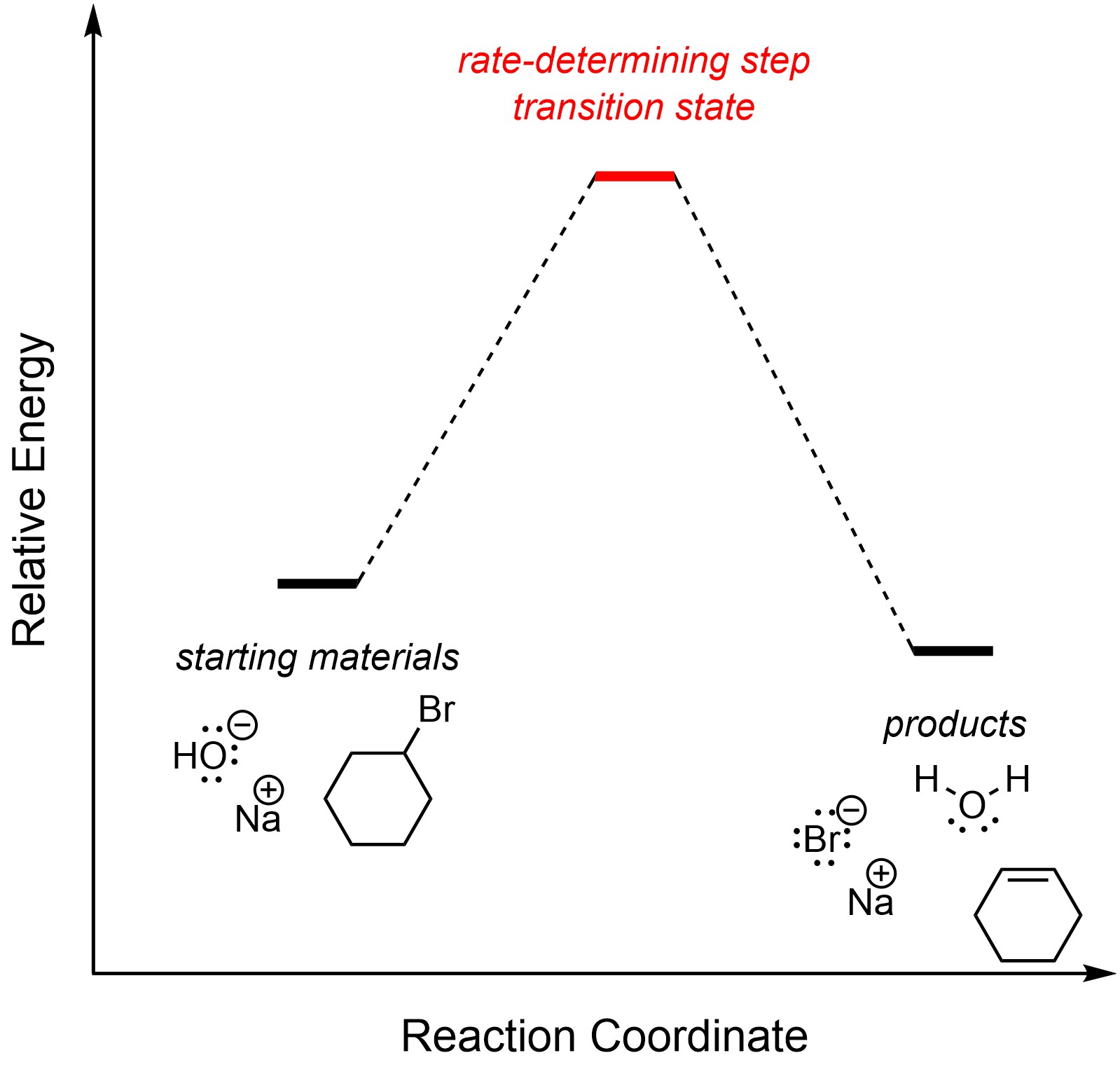
Figure 12.1 – Reaction Coordinate for E2 Elimination of Bromocyclohexane with Sodium hydroxide.
12.2.3. Anti-Bonding Orbital Requirements
Recall that when two orbitals overlap constructively they form a covalent bond (σ or π; see Section 1.3.1). However, when they overlap deconstructively they form an anti-bonding orbital (σ* or π*; see Section 1.3.1). Usually, in order to break a covalent bond electron density must be put into its corresponding anti-bonding orbital (Figure 12.2). It is only possible for the new π bond to form at the same time that both old bonds are broken by having the electron density from the old bond eclipse the anti-bonding orbital of the leaving group. This allows the electrons that previously made the C-H σ bond to immediately become the electrons of the new C-C π bond. As a result, the C-H and C-LG σ bonds being broken must be antiperiplanar to each other.
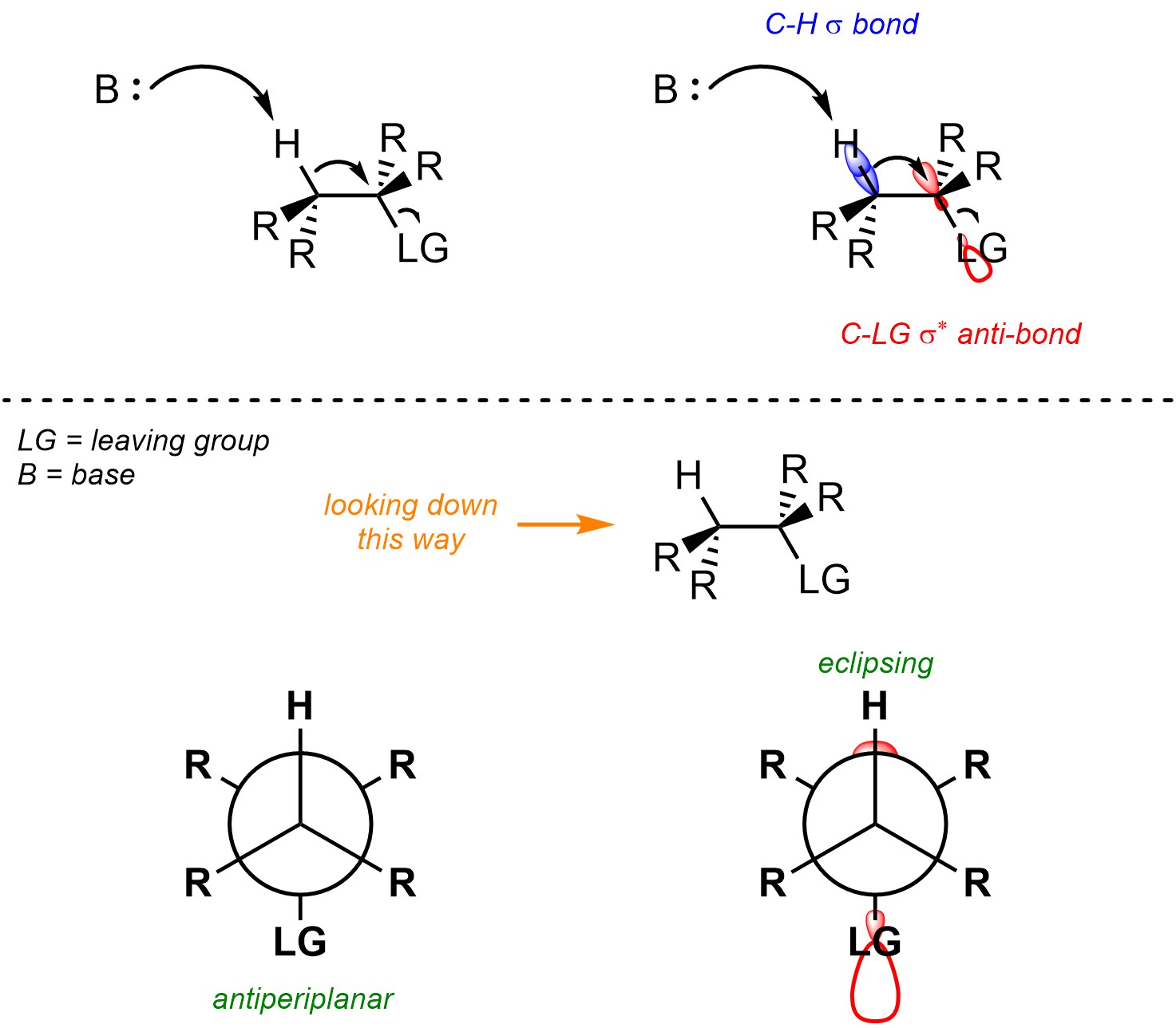
Figure 12.2 – Representation of the Geometric Constraint of the C-H σ Bond Eclipsing the C-LG σ* Anti-Bonding Orbital for E2 Reactions.
12.2.4. Regioselectivity – Zaitsev’s Rule
Many elimination reactions can form multiple products (regioisomers) depending on which hydrogen is removed with the leaving group (Scheme 12.3). Barring other factors (see Sections 12.2.4.1-12.2.4.3) the major regioisomer will be the one where the alkene has the most substituents.
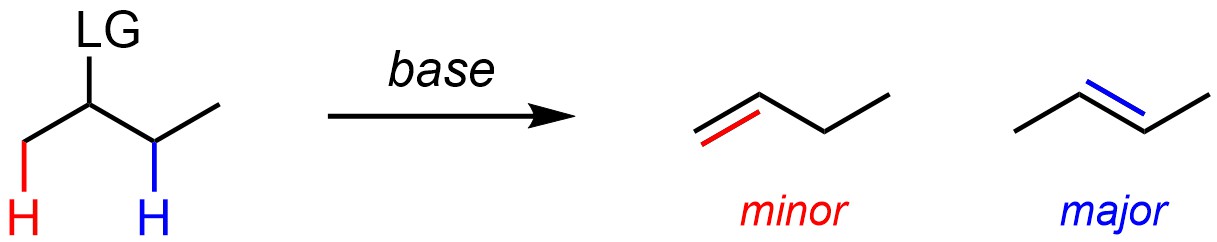
Scheme 12.3 – Regioselectivity in Elimination Reactions.
This is sometimes referred to as Zaitsev’s Rule (the major regioisomer formed will be the most thermodynamically stable one one [the one that is lower in energy]). Generally, the more substituents an alkene has the more thermodynamically stable it is. Why this is true is beyond the scope of this text. Technically there are many reactions that do not follow Zaitsev’s Rule. However, at an introductory level only the two main causes of exceptions will be considered (see Sections 12.2.4.2 and 12.2.4.3).
12.2.4.1. Effects from Resonance
Alkenes are more stabilized by having additional conjugation/delocalization and resonance forms than by having more substituents (Scheme 12.4). As a result, if conjugation is possible the major product will have it rather than additional substituents. This is consistent with Zaitsev’s Rule because it is the relative stability of the products that dictates regioselectivity.
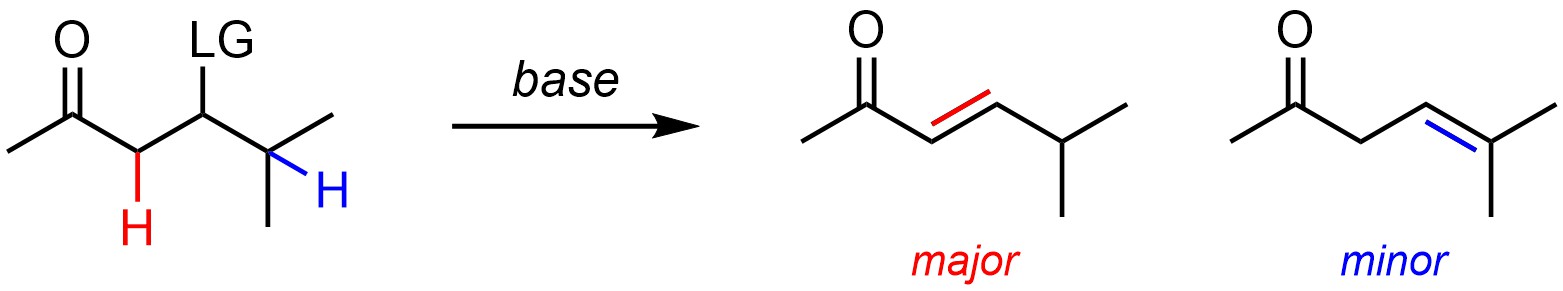
Scheme 12.4 – Regioselectivity in Elimination Reactions Where Conjugation is Possible.
12.2.4.2. Effects from Sterically Bulky Bases
If the base is very large, with a large amount of steric bulk/interactions, then regioselectivity can be reversed and the major regioisomer will be the one where the alkene has the fewest substituents (Scheme 12.5). The change in regioselectivity is the result of steric strain between the two molecules affecting the two possible acid-base reactions differently. This is almost always done by using a t-butoxide base. Other bases, with more complex structures, may work but are not normally discussed at an introductory level.
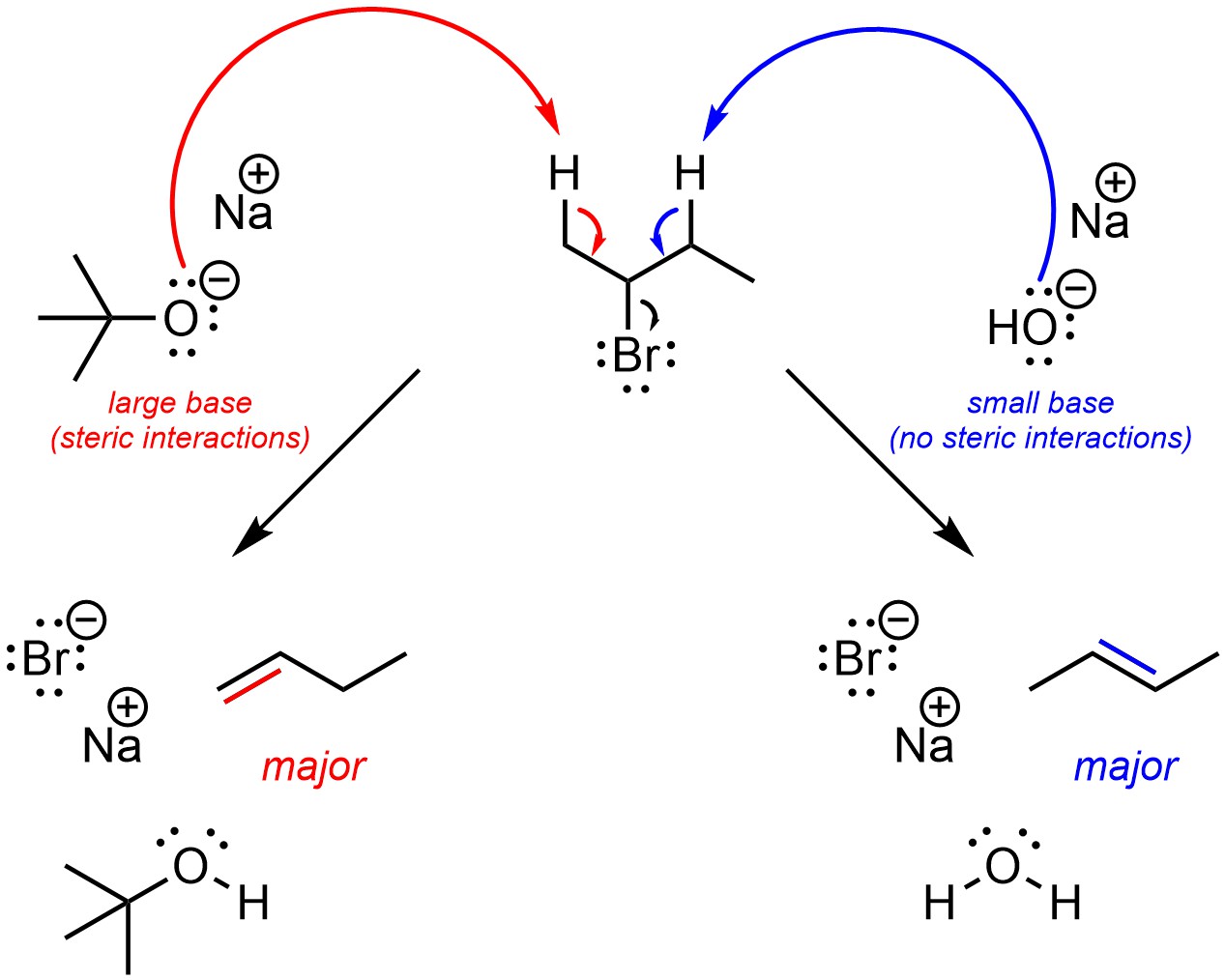
Scheme 12.5 – Example of the Change in Regioselectivity by Using Sodium Hydroxide, a Small Base, or Sodium t-Butoxide, a Large Base, for Selectively Undergoing One of Two Competing Reactions with 2-Bromobutane.
Often this approach does not work well; steric interactions normally do not affect acid-base reactions, which are a component of elimination mechanisms. At an introductory level, assume t-butoxide bases will change regioselectivity in elimination reactions.
12.2.4.3. Effects from Chair Conformations
By far the most important exceptions to Zaitsev’s Rule are in E2 elimination reactions involving six-membered rings. In order for E2 eliminations to occur the hydrogen and leaving group involved must be antiperiplanar to each other (see Section 12.2.3). This is only possible if the leaving group is axial (Scheme 12.6). In chair conformations where the leaving group is equatorial its corresponding σ* anti-bonding orbital points directly into the centre of the ring. As a result, it is not geometrically possible for any C-H σ bond to eclipse it.
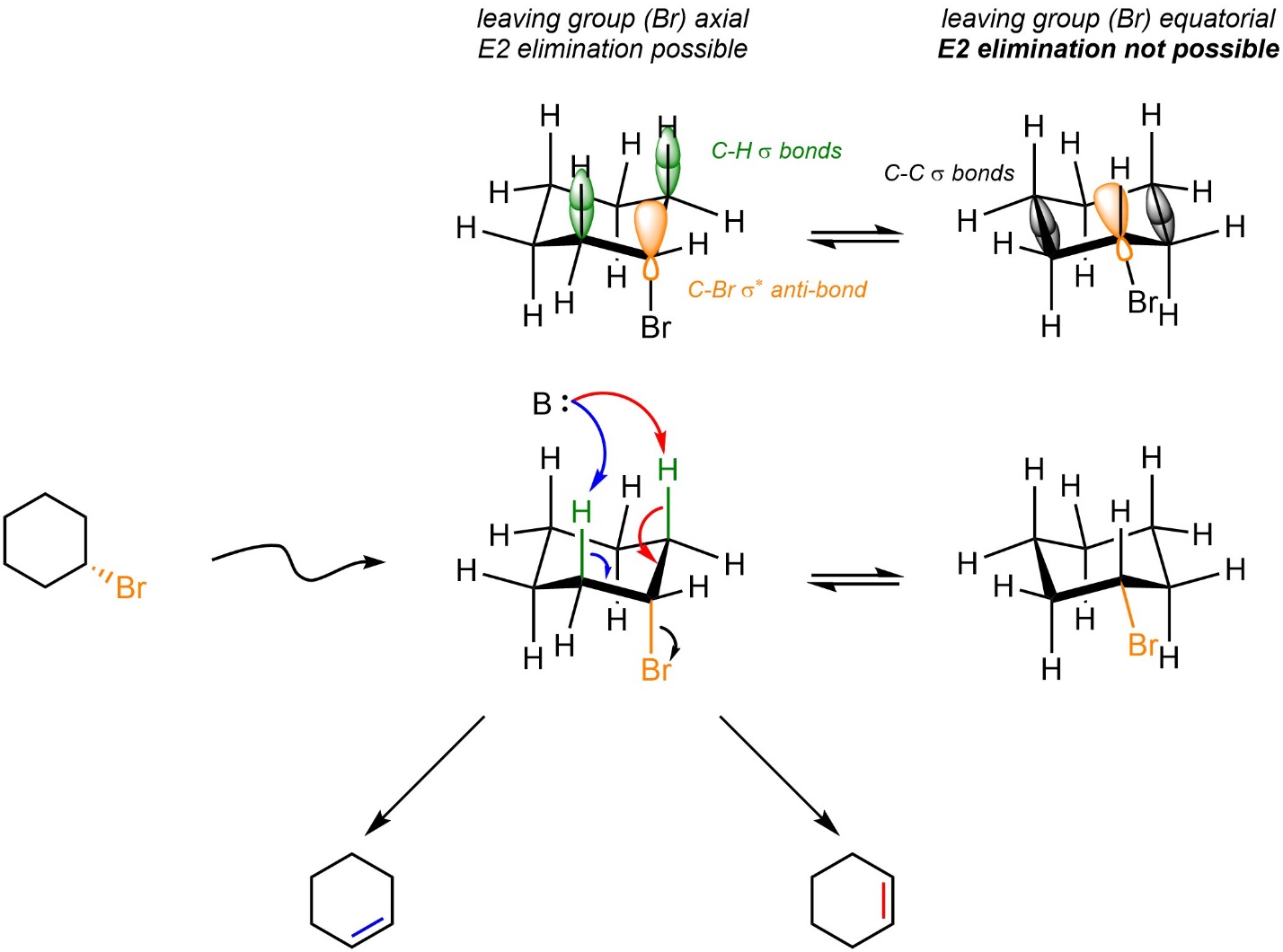
Scheme 12.6 – Orbital Overlaps Demonstrating Requirement for Leaving Group Being Axial in Six-Membered Rings.
Depending on the relative location and configuration of any other substituents on the ring it may or may not be possible for the more substituted alkene to be formed (Scheme 12.7). If it is possible, then that will still be the major product. However, if it is not possible then the E2 elimination still occurs but the other regioisomer is generated.
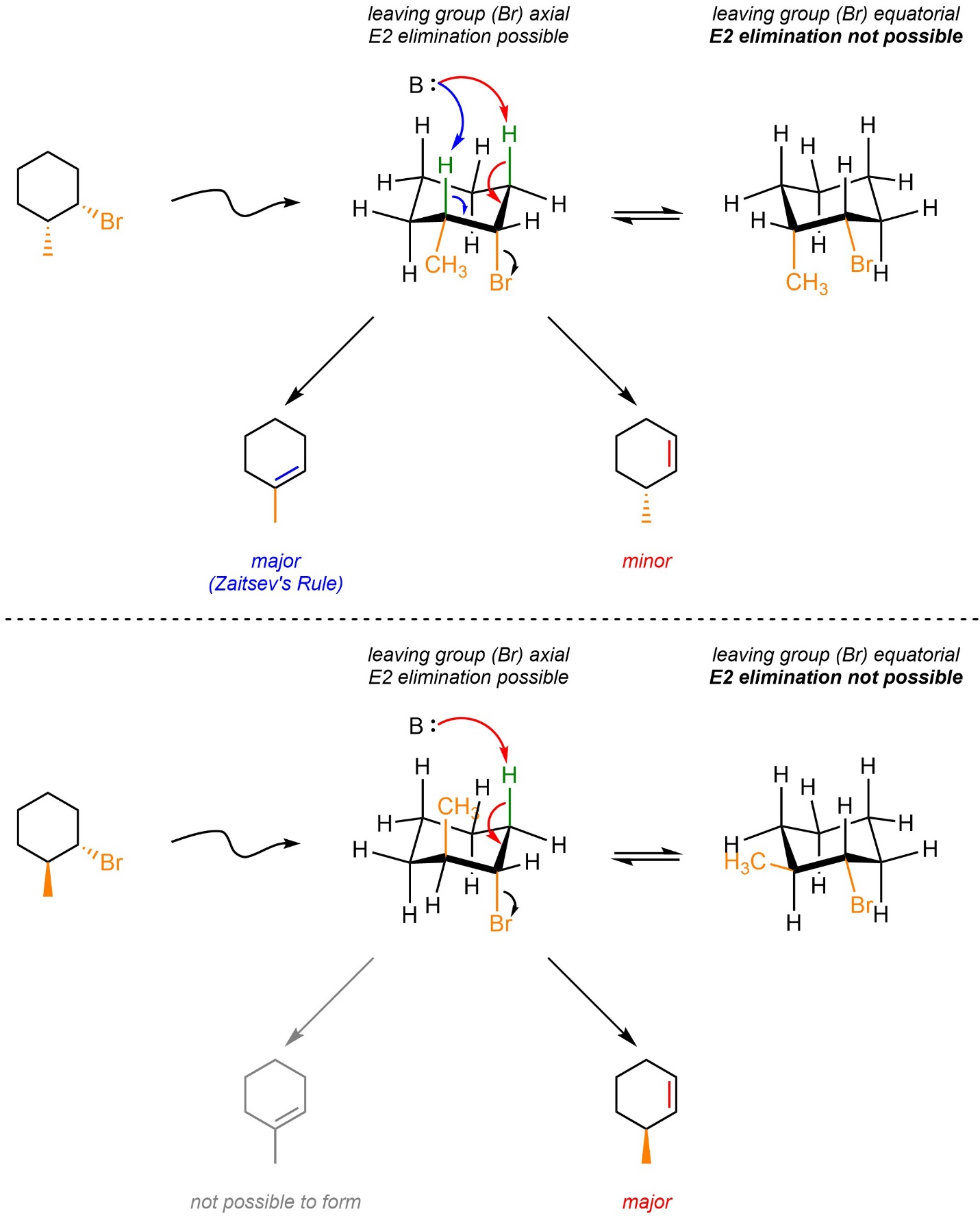
Scheme 12.7 – Example of Effects from Relative Configuration of Adjacent Groups on Regioselectivity for E2 Eliminations in Six-Membered Rings.
12.2.5. Stereoselectivity
Reactions that follow the E2 elimination mechanism are stereoselective for the formation of alkenes where the largest groups on either end of the alkene are trans relative to each other. Usually this means they selectively form E-alkenes. The stereochemistry of the alkene can be predicted by considering the Newman projections along the bond that will become the alkene (Figure 12.3).
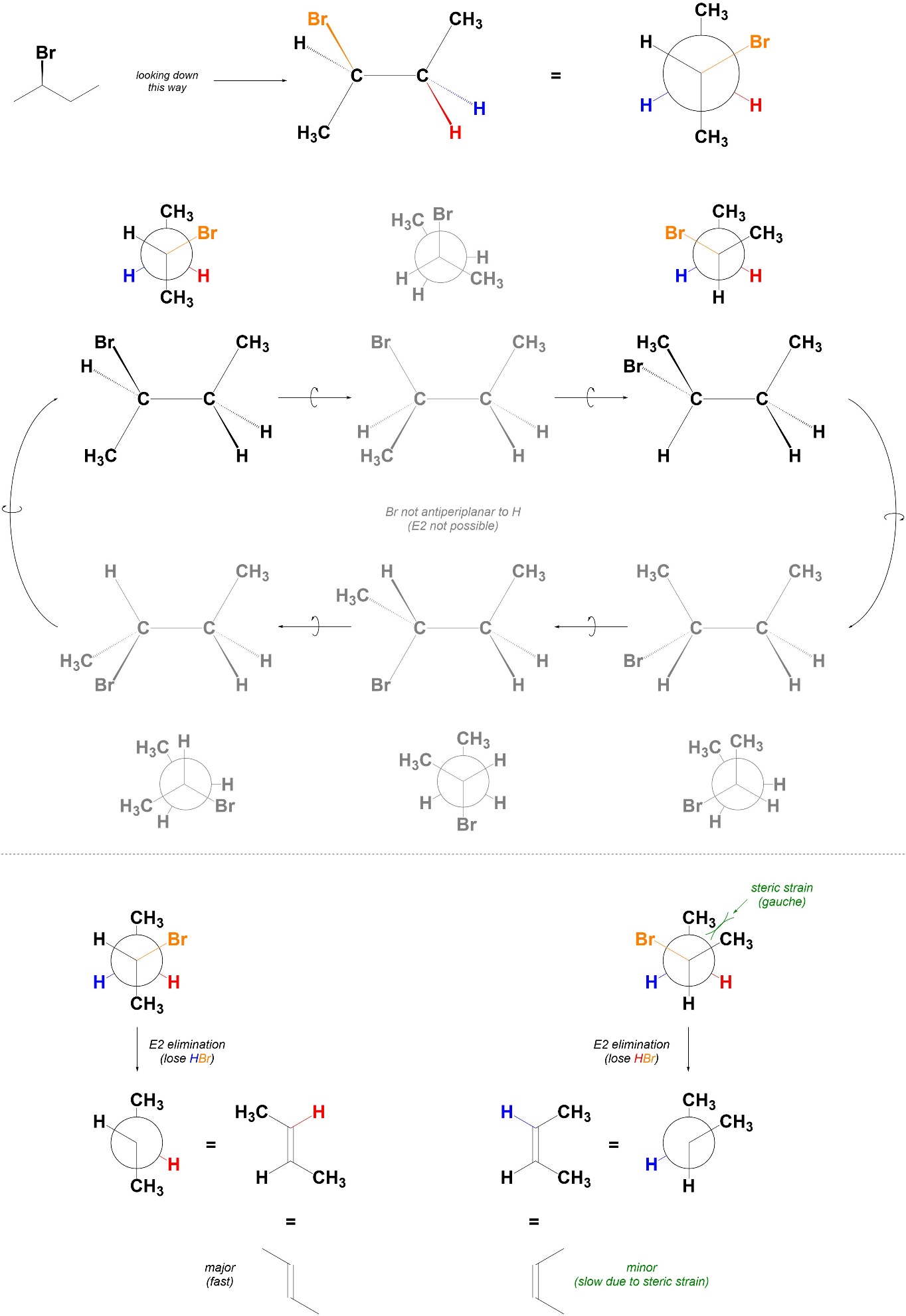
Figure 12.3 – Example of Determining Stereoselectivity in E2 Eliminations by Examining Possible Conformations.
Because E2 elimination is only possible when the C-H and C-LG bonds are antiperiplanar to each other, only certain conformations need to be considered. If there are multiple conformations to be considered, the most stable one will lead to the major product. This is the conformation with the smallest amount of steric/torsional strain.

