12.1. Addition vs. Elimination, SN2 and SN1 vs. E2 and E1
Using a variety of reaction conditions, it is possible to add two groups across an alkene (Scheme 12.1, see Chapter 8). Elimination reactions are simply the reverse of this, removing two groups to form an alkene.
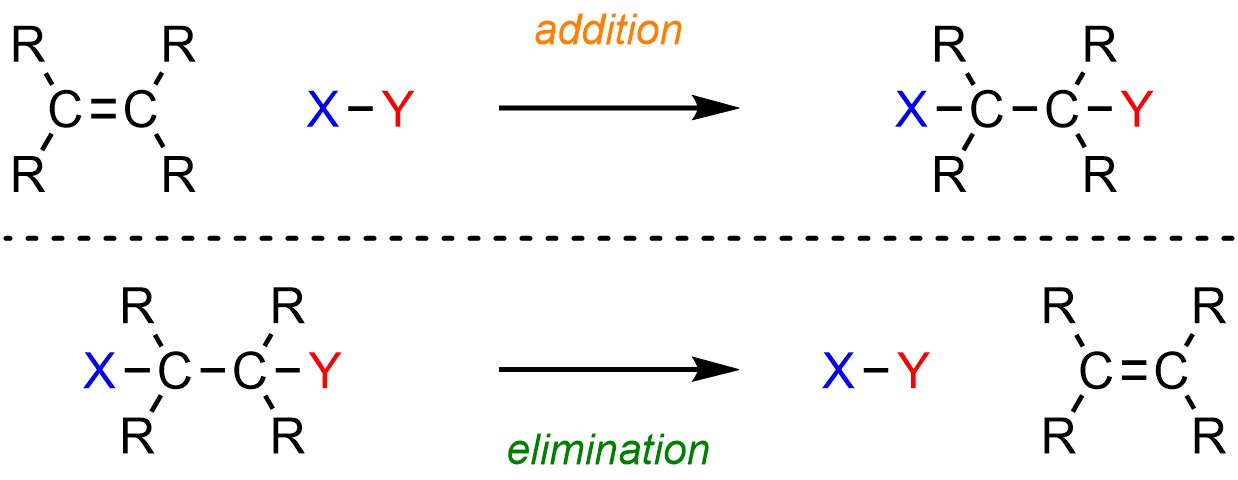
Scheme 12.1 – Generalized Reaction Equations for Electrophilic Addition Across an Alkene and Elimination to Form an Alkene.
Nucleophilic substitution reactions are classed into two broad categories depending on the mechanism they follow: SN2 reactions occur in one step and are stereospecific, SN1 reactions occur in two steps and are not stereospecific. Elimination reactions are classed in exactly the same way.
Most elimination reactions follow one of two possible mechanisms. Technically, more complex elimination mechanisms are possible and sometimes discussed in advanced texts, but these are specific variations of the two main mechanisms. Unlike with nucleophilic substitution reactions, it is often extremely difficult to predict which of the two mechanisms will be followed in a given reaction. The goal of this chapter is to discuss the two possibilities and give enough context that students can determine the products for elimination reactions. This text will specify which mechanism an elimination reaction follows or provide context to make it easily determinable.
There are many specifically named elimination reactions. For example, the formation of an alkene by removing a hydrogen and a halide (see Section 12.6) is sometimes called dehydrohalogenation. This text will generally avoid using the specific names, but it is possible that they may be encountered in assignments, labs, discussions, etc. In these cases, searching the name of the reaction using a resource such as Wikipedia will be a straightforward way of understanding what is being discussed.

