10.7. Reaction: Sulfonation
It is possible to change a hydrogen (H) into a sulfonic acid (SO3H) on an aromatic ring (Scheme 10.8). This is often referred to as sulfonation.
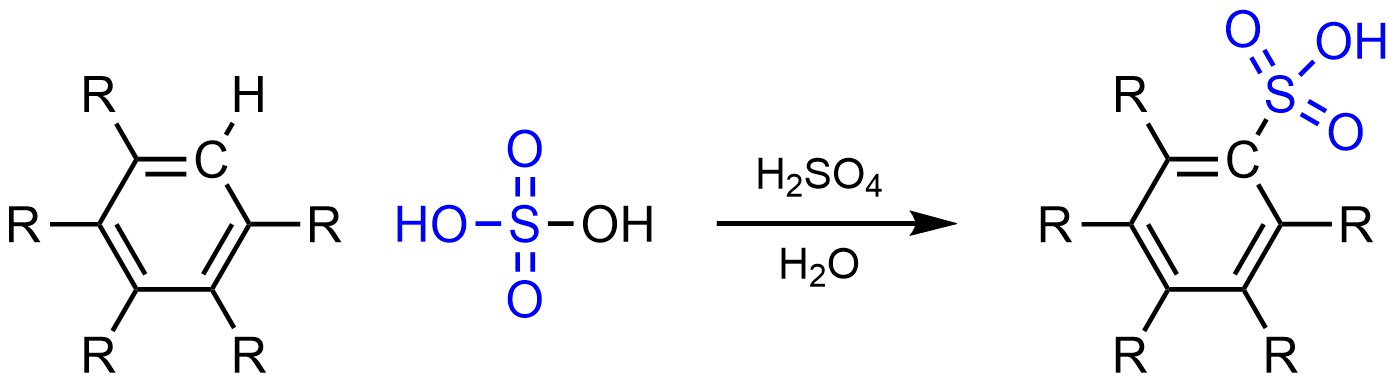
Scheme 10.8 – Generalized Reaction Equation for Sulfonation of an Aromatic Ring.
10.7.1. Catalyst Requirements
Sulfonation reactions are unusual in that the reagent used, sulfuric acid, is both a starting material and a catalyst for the reaction. No additional catalyst is needed. It is important to remember that sulfuric acid (H2SO4) is always contaminated with a small amount of water (H2O).
It is possible to do sulfonation reactions using sulfur trioxide (SO3) in sulfuric acid. The mechanism is overall the same, but the reaction is (much) faster. However, use of sulfur trioxide requires special conditions and equipment. As a result, this procedure is less common.
10.7.2. Mechanism
The overall mechanism for this reaction has the same two main steps but has additional activation steps (Scheme 10.9). The sulfuric acid reacts with water to form hydronium (not shown). This is the active catalyst. First, the electrophile (H2SO4) gets activated by the catalyst. It then ejects water and forms a sulfonyloxonium ion (HSO3+). This greatly increases its electrophilicity. Then, a π bond from the aromatic ring (nucleophile) attacks the sulfonyloxonium (activated electrophile). This creates a new C-S bond and a carbocation. Finally, water (nucleophile/base) removes the original hydrogen (electrophile/acid), which regenerates aromaticity and the catalyst. Some texts show the sulfuric acid directly reacting with itself to form the sulfonyloxonium. Since water is present, this is unlikely to be accurate.
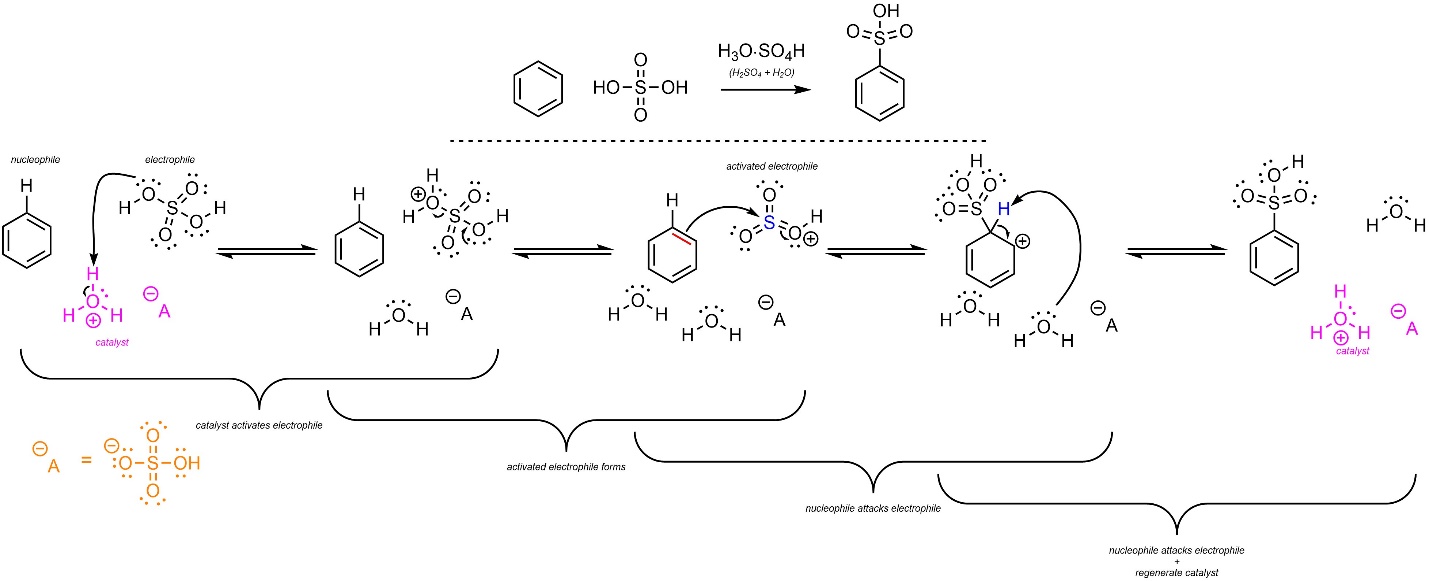
Scheme 10.9 – Reaction Mechanism for Acid-Catalyzed Sulfonation of Benzene.

