10.5. Reaction: Halogenation
It is possible to change a hydrogen (H) into a halogen (X; X = F, Cl, Br, I) on an aromatic ring (Scheme 10.4). This is often referred to as halogenation.
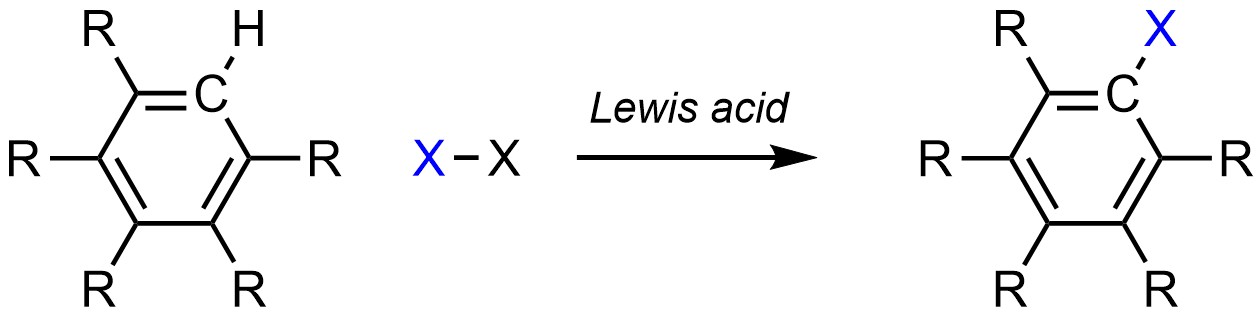
Scheme 10.4 – Generalized Reaction Equation for Halogenation of an Aromatic Ring.
10.5.1. Catalyst Requirements
Other than fluorine (F2), directly using a dihalide (Cl2, Br2, I2) will not result in halogenation without a catalyst. The catalysts used are Lewis acids, and work by coordination with the dihalide (Table 10.1). The use of copper chloride instead of iron or aluminum iodide for the addition of iodine is due to their instability rather than any catalytic difference between them.
Table 10.1 – Common Reagents for Halogenation of Aromatic Rings.
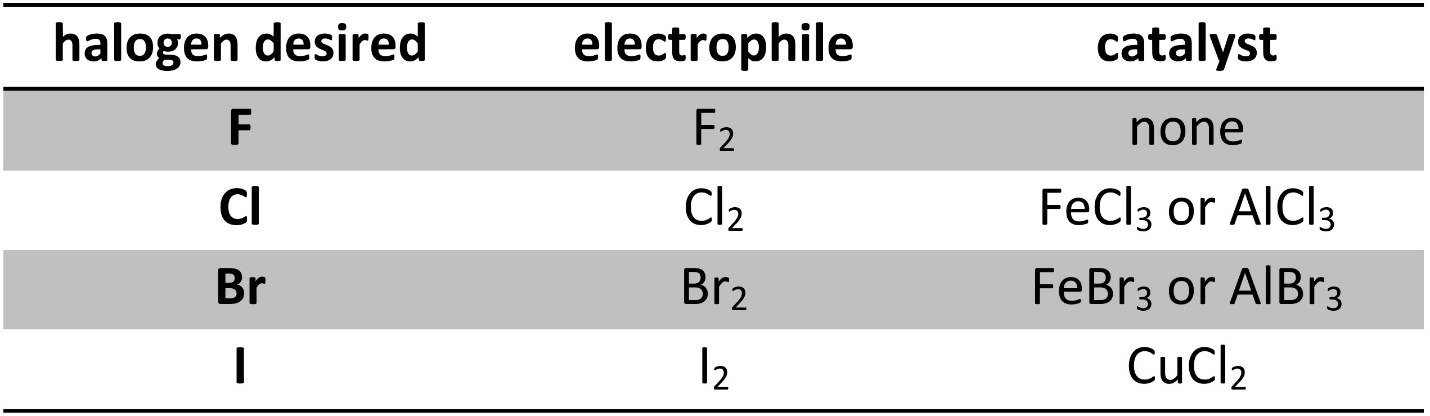
It is possible to halogenate aromatic rings using other electrophiles and catalysts, but these reagents are typically more complex.
10.5.2. Mechanism
The overall mechanism for this reaction has the same two main steps but has an additional activation step for the catalyst (Scheme 10.5). The dihalide (nucleophile) reacts with the Lewis acid catalyst (electrophile) to form an ionic complex. This greatly increases its electrophilicity. Then, a π bond from the aromatic ring (nucleophile) attacks the halogen (activated electrophile). This creates a new C-X bond and a carbocation. Finally, the other halide (nucleophile/base) removes the original hydrogen (electrophile/acid), which regenerates aromaticity and the catalyst. Although halides are very weak bases, the last step is favourable because it regenerates aromaticity.
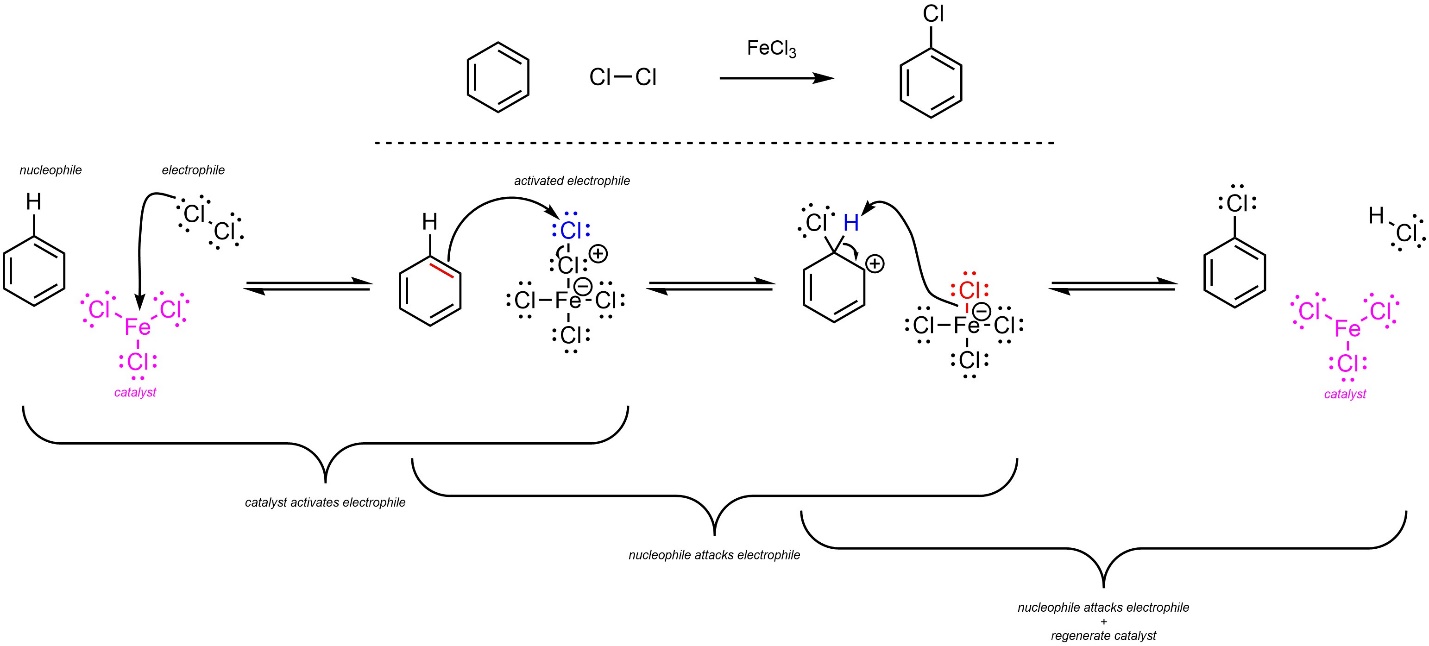
Scheme 10.5 – Reaction Mechanism for Lewis Acid-Catalyzed Halogenation of Benzene Using Chlorine and Iron Trichloride.

