10.1. (Very) Brief Refresher of the Basics
Recall that carbon-carbon π bonds such as those in alkenes are nucleophilic (see Chapter 8). For example, they may attack electrophiles to generate carbocation intermediates, which are then electrophiles and attacked by another nucleophile (Scheme 10.1).
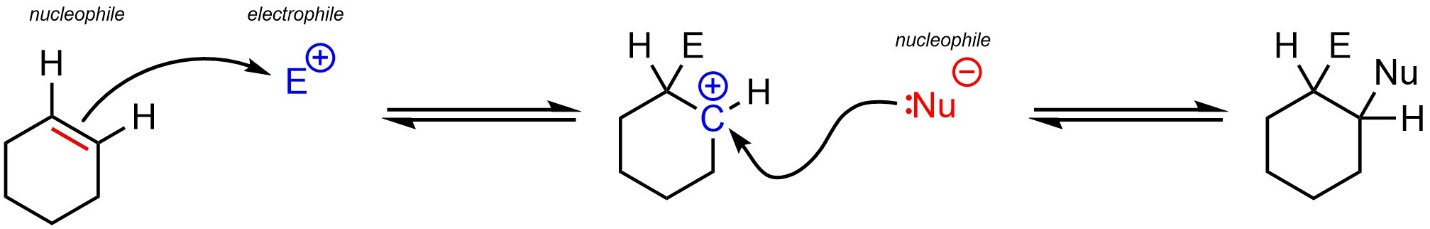
Scheme 10.1 – Generic Example of a Nucleophilic Attack from an Alkene.
This (and related mechanisms) is the basis for reactions such as halogenation of alkenes. Recall that these reactions work well for alkenes, but do not work for the π bonds of aromatic rings (see Scheme 9.1) because this would remove aromaticity and eliminate the aromatic stabilization energy the molecule gains from it.

