1.1. (Very) Brief Refresher of the Basics
A quick reminder of some of the material you will have encountered before will help keep the rest of the chapter in context. Most of Section 1.1 should be a review, and as a result will not be directly tested on. However, it is very important to ensure these concepts are well understood as new material will build directly on them.
1.1.1. The Periodic Table
The Periodic Table represents one way of organizing the known elements based on their properties. While a full Periodic Table contains many useful pieces of information most of it is not relevant to a discussion of the basics of Organic Chemistry. An abridged Periodic Table (Figure 1.1) with the atomic symbols, element numbers, and Group numbers (column numbers) is sufficient in the first half of this text. Notice that, depending on which Periodic Table you use, the Group numbers may be different. For all references to Group numbers in this text, please use these values.

Figure 1.1 – Abridged Periodic Table of the Elements.
1.1.2. Atomic Orbitals
Recall that electrons occupy regions of space called orbitals. Atomic orbitals have predefined shapes that can be determined from their corresponding quantum numbers and the use of a wavefunction. These atomic orbitals represent the area around the nucleus where the electrons in it are most likely to be (a probability distribution).
The simplest atomic orbitals are s orbitals, which look like spheres (Figure 1.2). For our purposes the presence of nodes in s orbitals can be ignored.
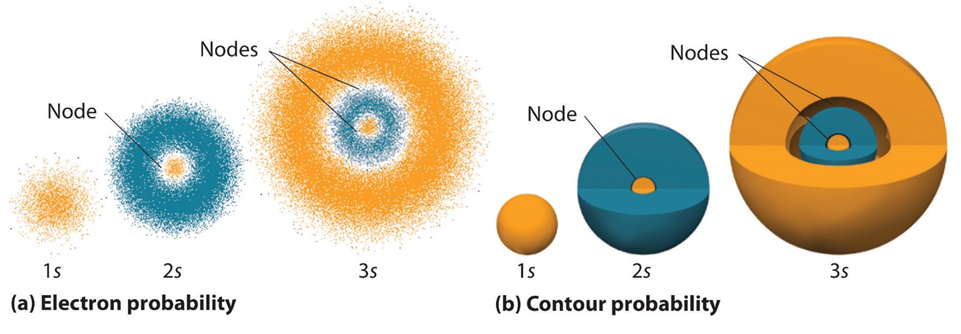
Figure 1.2 – Probability Densities for the 1s, 2s, and 3s Orbitals of the Hydrogen Atom.
The next atomic orbitals are p orbitals, which are often compared to dumbbells (Figure 1.3). There is a nodal plane that splits the orbitals into two differently phased lobes on either side of the nucleus. “Phase” represents the sign of the function for that area (+ or -) and is not physically meaningful, nor does it have any relationship to charge. It does however play a crucial role in bonding.
As a result of the quantum numbers used to derive them, there are 3 equivalent p orbitals for each level: px, py, and pz. These three orbitals are orthogonal (perpendicular with respect to each other) and most easily viewed as aligned with the coordinate axes.
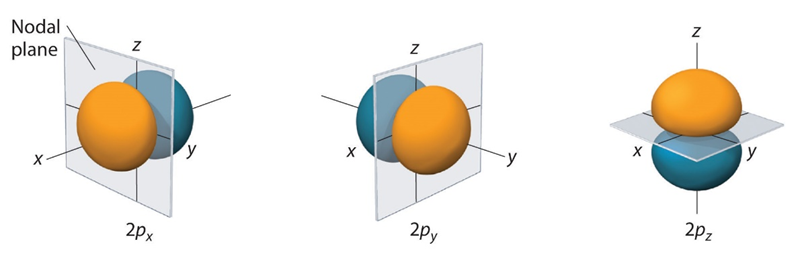
Figure 1.3 – The Three Equivalent 2p Orbitals of the Hydrogen Atom.
Other types of atomic orbitals (d, f, g, h, i) can be defined, but are not normally considered/occupied in simple organic systems
1.1.3. The Aufbau and Pauli Exclusion Principles
As a result of their spin quantum number and the Pauli Exclusion Principle (no two electrons can have the same four quantum numbers) each orbital may hold a maximum of two spin-paired electrons. This means an s orbital may hold up to two electrons, and each of the three p orbitals (px, py, and pz) may also hold up to two electrons.
In any given system most things like to rest in the lowest possible energy state. The Aufbau Principle describes the order in which electrons fill orbitals in order of increasing energy. An arrowed diagram (Figure 1.4) is the most common way to depict the order the ascending energy levels take.
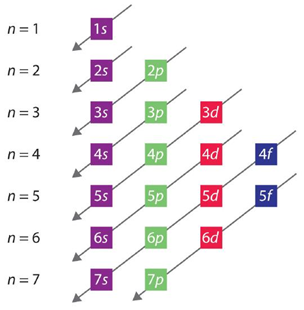
Figure 1.4 – Order in Which Orbitals Are Filled in Multielectron Atoms.
1.1.4. Orbital Energies
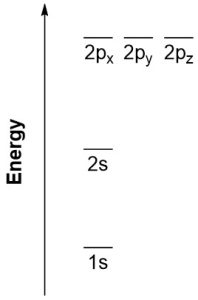
Based on the order of ascending orbital energies (Figure 1.4) electrons first fill the lowest energy orbital (1s), then the next lowest (2s), then the next lowest (2p), and so on (Figure 1.5). It is important to remember that atomic orbitals of the same type also have the same energy. For instance, the 2p orbitals are the 2px, 2py, and 2pz orbitals, all of which have exactly the same energy level. A set of orbitals with exactly the same energies is sometimes referred to as being degenerate.
Figure 1.5 – Simple Energy Diagram for 1s, 2s, and 2p Orbitals.
1.1.5. Valence vs. Core Electrons and Orbitals
Orbitals in systems like atoms or molecules can be broadly divided into two categories. The fully occupied orbitals of the lowest energies are referred to as core orbitals, and the electrons in them as core electrons. These orbitals and electrons are low in energy (stable) and close to the nucleus. As a result, they are not normally considered important for bonding or chemical reactivity and are typically ignored. Conversely, the highest energy orbitals (vacant, semi-occupied, or fully occupied) are referred to as valence orbitals. Any electrons in these orbitals are called valence electrons. These orbitals and electrons are higher in energy (less stable) and farther from the nucleus. As a result, they are heavily involved in bonding and chemical reactivity.
Core orbitals: fully occupied orbitals of lowest energy
Core electrons: electrons found in core orbitals
(NOT available for bonding / as lone pairs)
(We can ignore them)
Valence orbitals: occupied orbitals of highest energy(and any accompanying empty orbitals of similar energy)
Valence electrons: electrons found in valence orbitals
(Available for bonding / as lone pairs)
Consider the ground-state electron configuration for carbon: 1s22s22p2 (Figure 1.6). The 1s orbital would be considered a core orbital, and the two electrons in it core electrons. The 2s, 2px, 2py, and 2pz orbitals would be considered valence orbitals and the four electrons in them valence electrons.
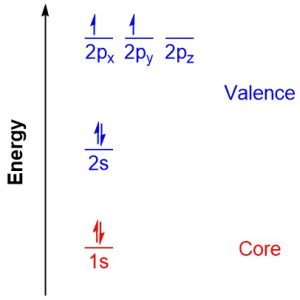
Figure 1.6 – Energy Diagram for Electron Configuration of Carbon in the Ground-State.
Notice that carbon has 4 valence electrons and is in Group 4 in the Periodic Table. This pattern holds for all atoms in the first few rows of the table; the number of valence electrons for an atom is equal to its Group number in the Periodic Table.
1.1.6. Ionic vs. Covalent Bonding
There are two primary forms of chemical bonding between atoms.
Ionic bonds typically occur when a valence electron is fully transferred from one atom’s valence orbital to another’s. The loss of an electron makes that atom cationic (positively charged). The gain of an electron makes the other atom anionic (negatively charged). The two charged species are then attracted towards each other, forming a tight bond. These types of compounds are commonly referred to as salts (Figure 1.7).
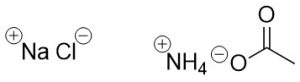
Figure 1.7 – Examples of Ionic Bonding in Simple Molecules.
Covalent bonds occur when valence electrons are shared between atoms. Two semi-occupied valence orbitals overlap and combine to form a new fully occupied molecular orbital (Figure 1.8). The bond can be thought of as an area between the two nuclei where the two electrons are being shared between them.
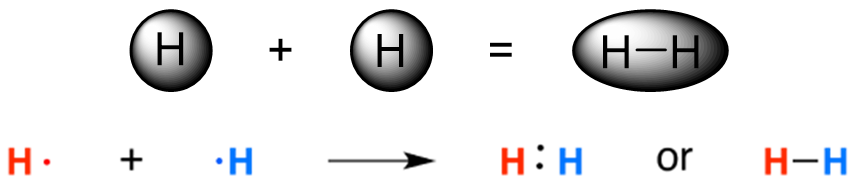
Figure 1.8 – Formation of a Covalent Bond Between Two Hydrogen Atoms.