10.6. Reaction: Nitration
It is possible to change a hydrogen (H) into a nitro group (NO2) on an aromatic ring (Scheme 10.6). This is often referred to as nitration.
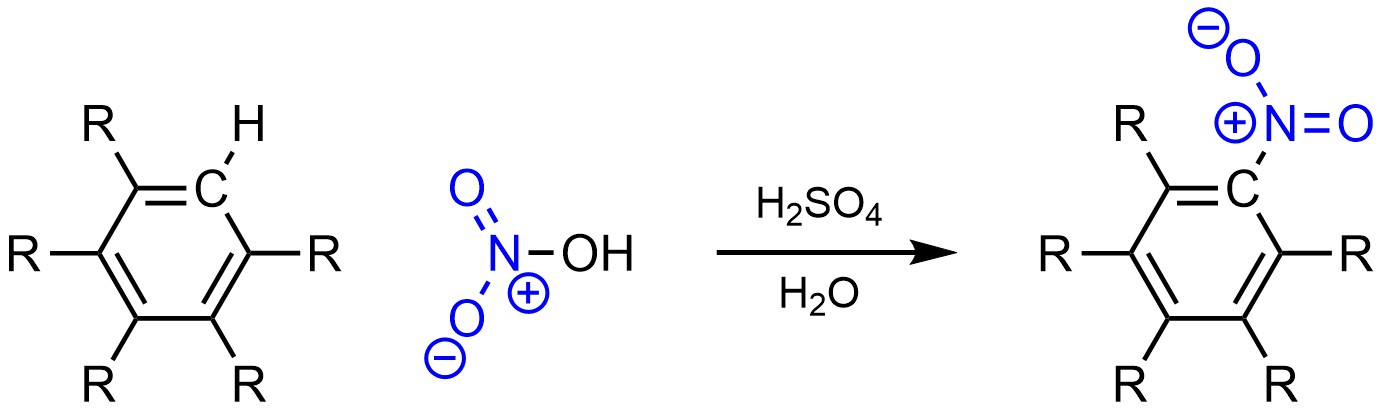
Scheme 10.6 – Generalized Reaction Equation for Nitration of an Aromatic Ring.
10.6.1. Catalyst Requirements
Directly using nitric acid (HNO3) will not result in nitration without a catalyst. The catalyst used is sulfuric acid (H2SO4) which always comes contaminated with a small amount of water (H2O).
10.6.2. Mechanism
The overall mechanism for this reaction has the same two main steps but has additional activation steps (Scheme 10.7). The sufuric acid reacts with water to form hydronium (not shown). This is the active catalyst. First, the electrophile (HNO3) gets activated by the catalyst. It then ejects water and forms a nitronium ion (NO2+). This greatly increases its electrophilicity. Then, a π bond from the aromatic ring (nucleophile) attacks the nitronium (activated electrophile). This creates a new C-N bond and a carbocation. Finally, water (nucleophile/base) removes the original hydrogen (electrophile/acid), which regenerates aromaticity and the catalyst. Some texts show the sulfuric acid directly reacting with the nitric acid to form the nitronium. Since water is present, this is unlikely to be accurate.
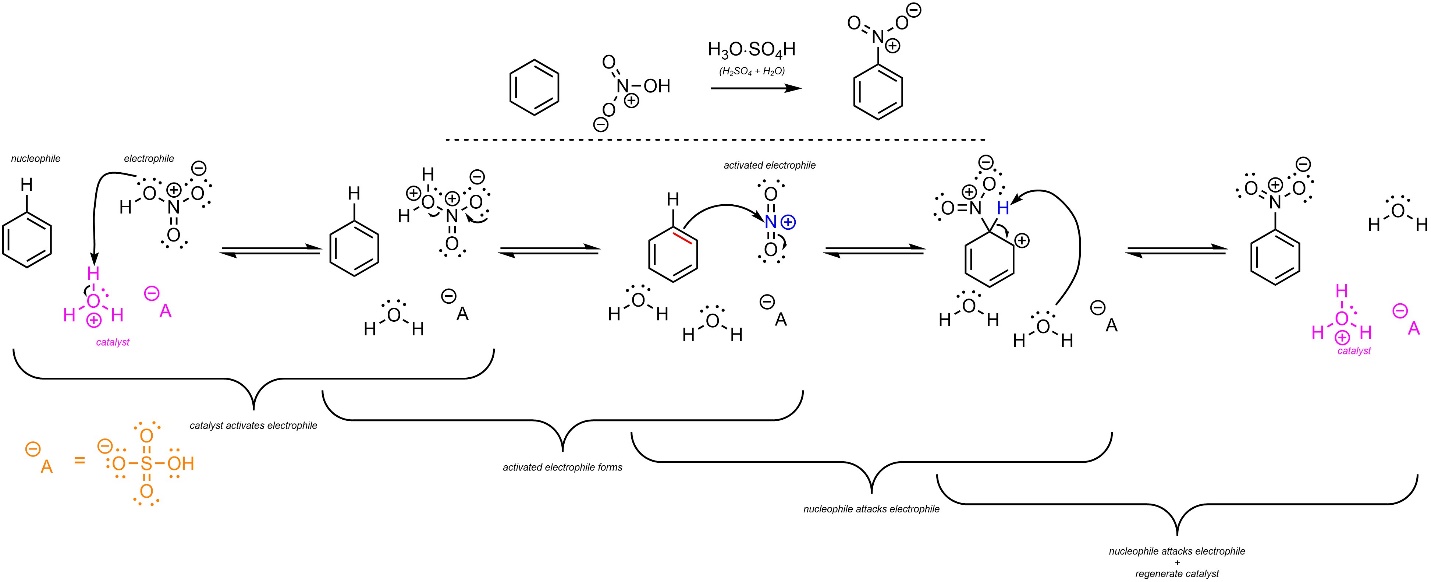
Scheme 10.7 – Reaction Mechanism for Acid-Catalyzed Nitration of Benzene.

