7.6. Additions of Organometallics to Pi (π) Bonds
Organometallics are a broad class of compounds where at least one carbon atom is bonded to a metal. The reactivity and properties of organometallics are complex, and most institutions have one or more entire courses dedicated to their study. At an introductory level only very simple organometallic compounds are discussed.
Most organometallic compounds have covalent bonds between the metal and carbon. However, the bond between them is often very polar (Figure 7.13). At an introductory level it is convenient to pretend that the bond is ionic, with an anionic carbon (carbanion) and a cationic metal.
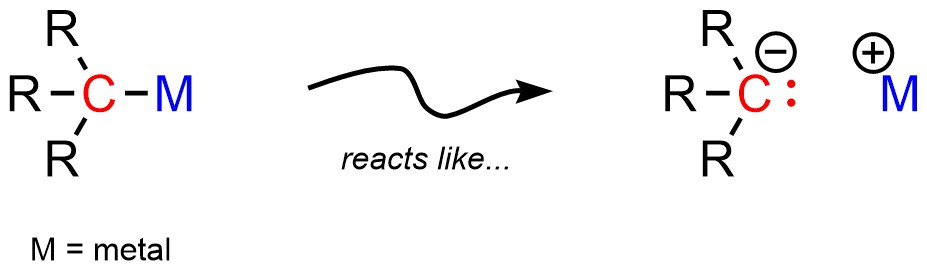
Figure 7.13 – Representations of Simple Organometallic Compounds.
Alkanes, alkenes, alkynes, and carbon-based aromatic rings are extremely weak acids (see Figure 6.5). As a result, carbanions are both very strong bases and very strong nucleophiles. Mechanisms for nucleophilic attacks of organometallic compounds onto electrophiles are, in reality, incredibly complex. Often radical/single-electron transfer steps occur, and the exact mechanism can change depending on the solvent, temperature, metal atom, structure of the electrophile, structure of the nucleophile, what other salts are added to the reaction mixture, etc. While the specifics of the reaction mechanism are complex, they can be approximated as simple nucleophile-electrophile attacks.
Because organometallic compounds are both powerful nucleophiles and powerful bases, special care must be taken when attempting to use them as nucleophiles. All acidic compounds, including water and alcohols, must be rigorously excluded from the reaction. For some organometallic compounds trace amounts of water can lead to problems, where instead of the desired nucleophilic reaction the organometallic reagent reacts rapidly as a base with the water and is consumed. Other compounds, such as organolithiums (see Section 7.5.2.), are so reactive that they will spontaneously ignite on contact with the moisture in the atmosphere. Experiments with these substances are normally reserved for students in upper-level courses.
7.6.1. Grignard Additions
Two types of simple organometallic compounds are very common. The less reactive of the two are Grignard reagents (sometimes abbreviated as Grignard or Grignards). All Grignard reagents have a carbon covalently bonded to a magnesium, which is also covalently bonded to a halogen (Figure 7.14). The halogen is most commonly bromine, though chlorine or iodine are also possible.
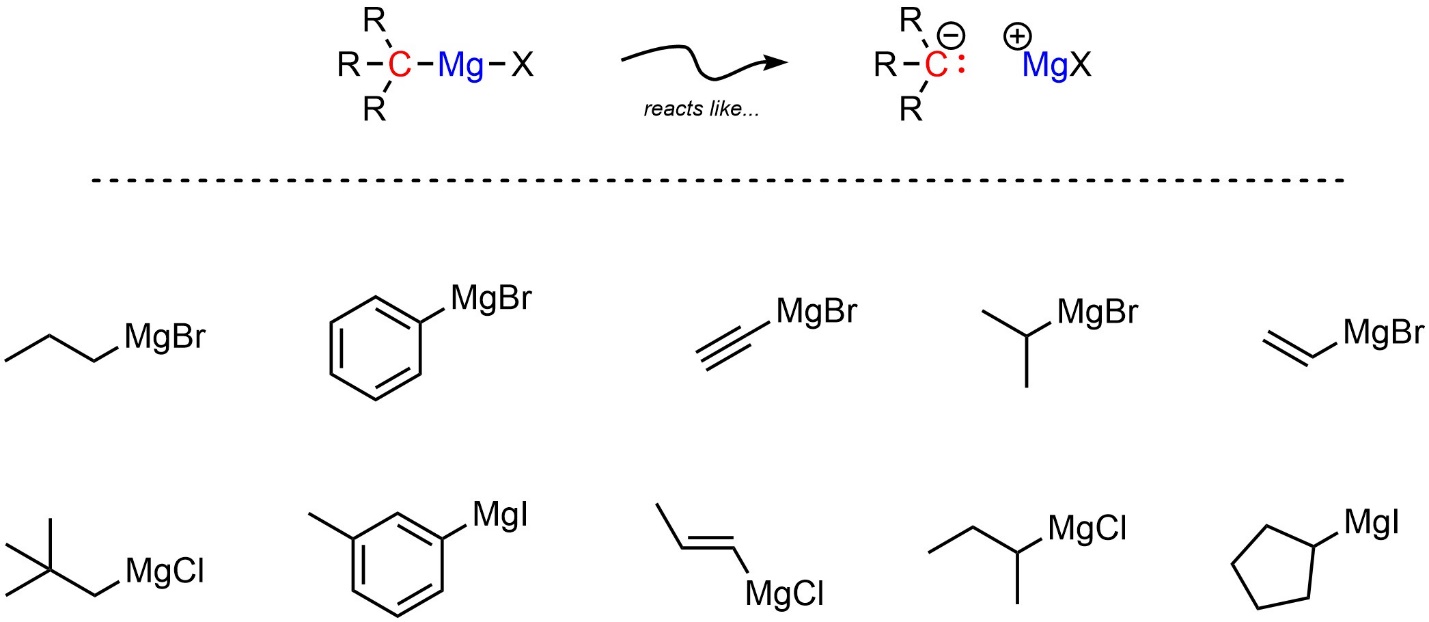
Figure 7.14 – Representation and Examples of Grignard Reagents.
Grignard reagents are typically formed from the addition of elemental magnesium (pure Mg) to an organohalide (Scheme 7.12). The exact mechanism for how this occurs is beyond the scope of this text. Importantly, Grignard reagents need to be formed and reacted in solvents that contain an ether functional group. Common solvents for this include diethyl ether (Et2O) and tetrahydrofuran (THF). Without a solvent that contains an ether functional group Grignard reagents are unstable and will rapidly decompose. The exact reasons for this requirement are again beyond the scope of this text.
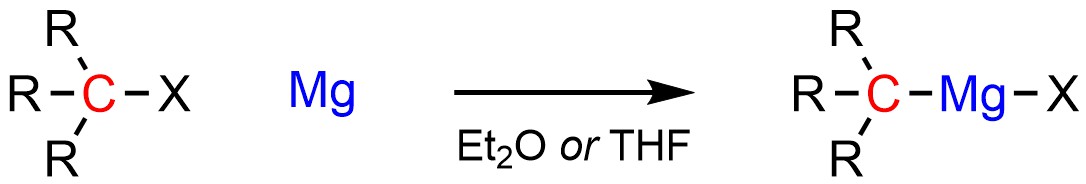
Scheme 7.12 – Formation of Grignard Reagents Using an Organohalide and Magnesium.
Grignard reagents can act as nucleophiles, attacking a wide variety of electrophiles including carbonyl-containing functional groups (Scheme 7.13). This reaction must be quenched using a weak acid to break the O-Mg bond and generate the final product. The overall reaction is often depicted as two steps to indicate that this is required. The mechanism for the quenching step is simple but requires additional information to discuss.
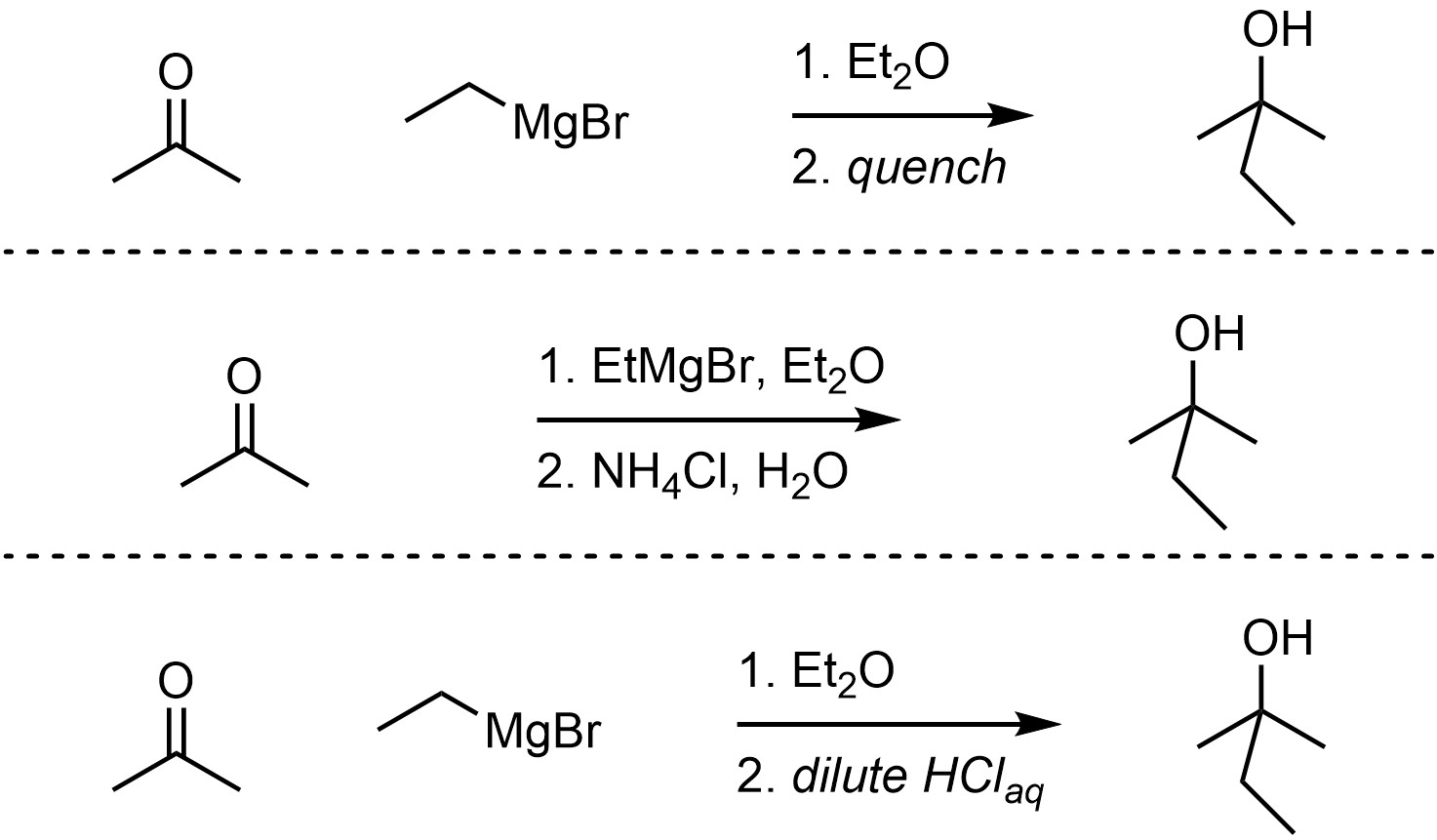
Scheme 7.13 – Examples of Ways of Depicting Overall Reactions for Grignard Reactions.
7.6.1.1. “Mechanism” of Grignard Addition Reactions
A mechanism for nucleophilic attacks of Grignard reagents onto carbonyl-containing electrophiles can be approximated by assuming they behave as carbanions (Scheme 7.14). Whether the O-Mg bond is covalent or ionic is debatable. At an introductory level showing it as either covalent or ionic is acceptable.
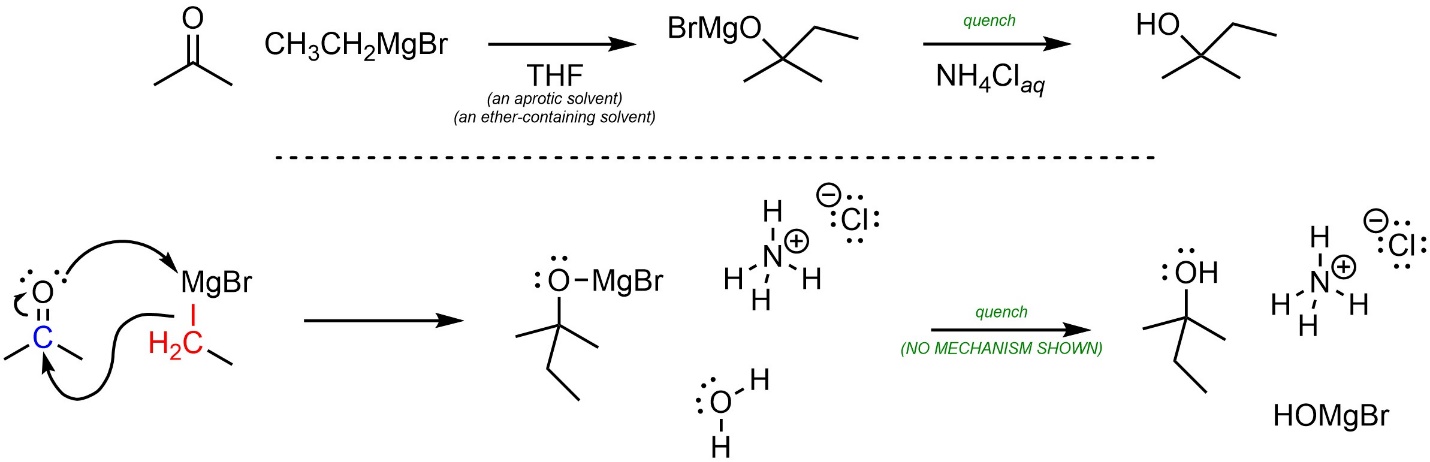
Scheme 7.14 – Approximated Mechanism for Nucleophilic Attack of Ethylmagnesium Bromide onto Acetone.
Again, it is important to remember that this is an approximation. Some sources may show other, more complex mechanisms for these reactions.
7.6.2. Organolithium Additions
Two types of simple organometallic compounds are very common. The more reactive of the two are organolithium reagents (sometimes abbreviated as organolithiums). All organolithiums have a carbon covalently bonded to a lithium (Figure 7.15).
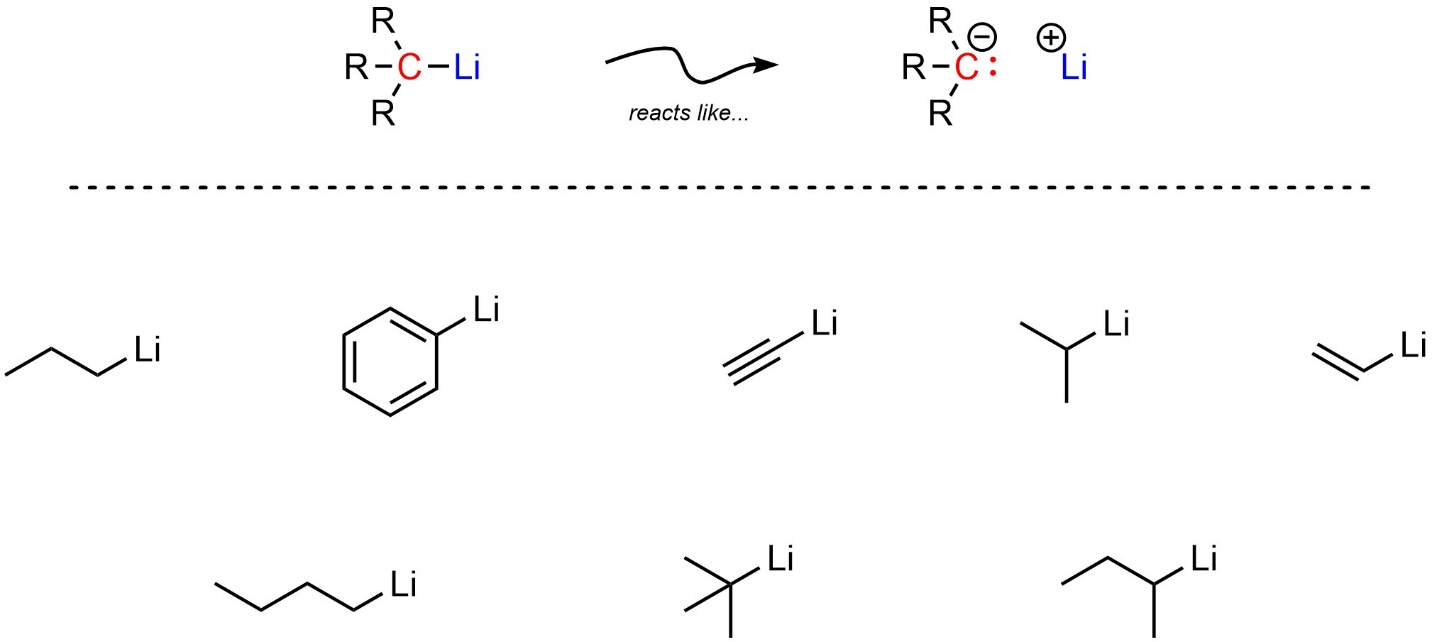
Figure 7.15 – Representation and Examples of Organolithium Reagents.
Organolithium reagents are often formed from the addition of (excess) elemental lithium (pure Li) to an organohalide (Scheme 7.15). The exact mechanism for how this occurs is beyond the scope of this text. Unlike Grignard reagents, they do not need to be formed and reacted in solvents that contain an ether functional group. However, it is common to use an ether-containing solvent for these reactions as they are easily available polar aprotic solvents.
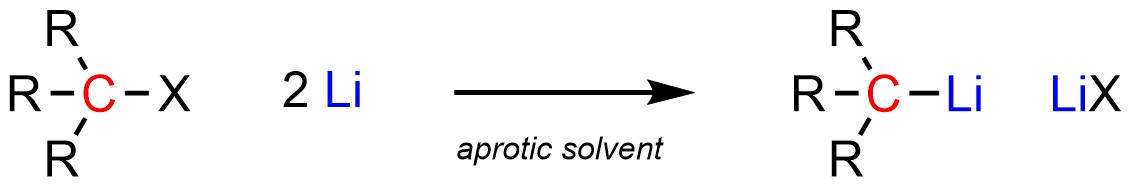
Scheme 7.15 – Formation of Organolithium Reagents Using an Organohalide and Lithium.
Organolithium reagents can act as nucleophiles, attacking a wide variety of electrophiles including carbonyl-containing functional groups (Scheme 7.16). This reaction must be quenched using water and/or a weak acid to break the O-Li bond and generate the final product. The overall reaction is often depicted as two steps to indicate that this is required. The mechanism for the quenching step is simple but requires additional information to discuss.
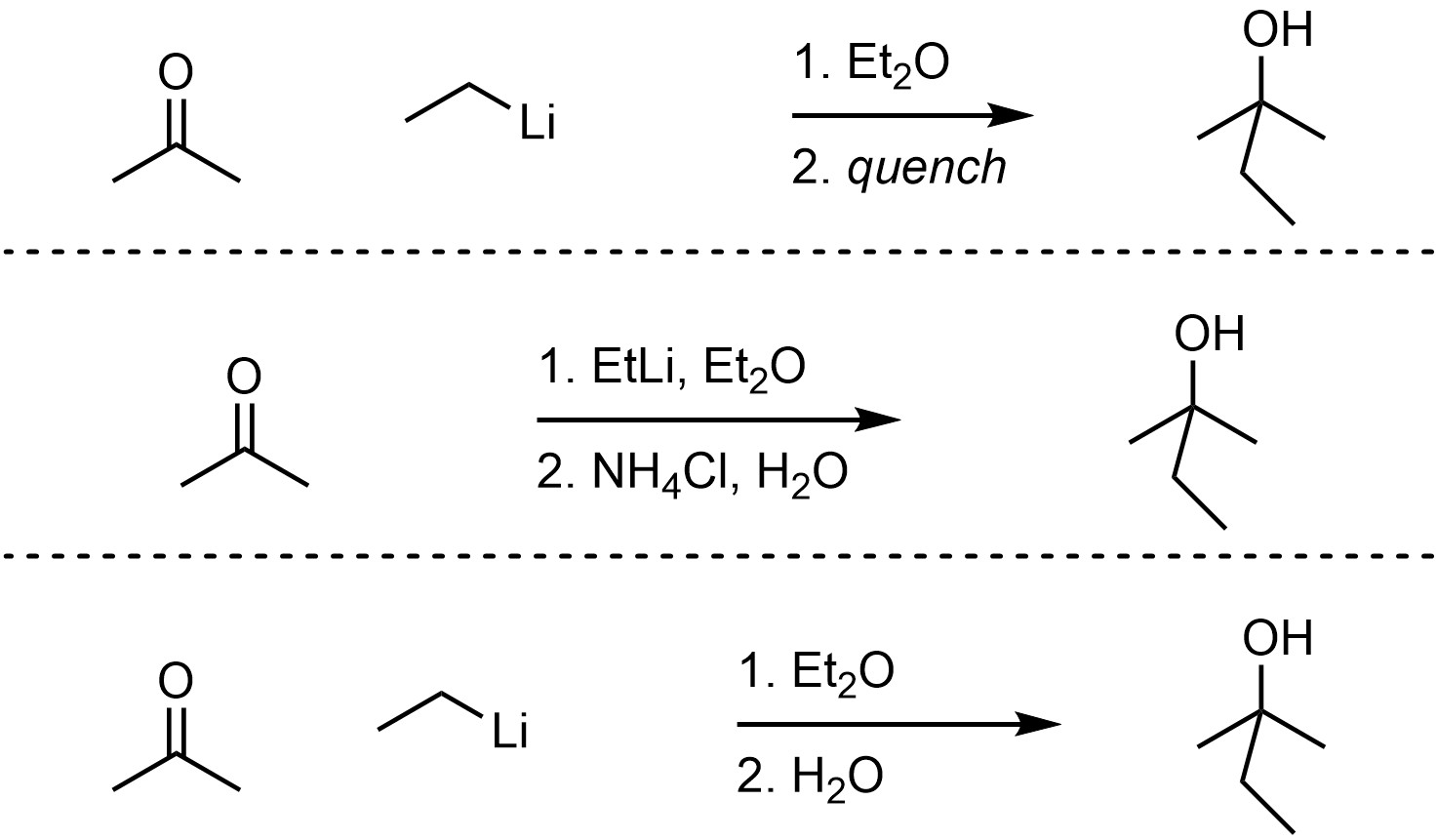
Scheme 7.16 – Examples of Ways of Depicting Overall Reactions for Organolithium Reactions.
7.6.2.1. “Mechanism” of Organolithium Addition Reactions
A mechanism for nucleophilic attacks of organolithium reagents onto carbonyl-containing electrophiles can be approximated by assuming they behave as carbanions (Scheme 7.17). Whether the O-Li bond is covalent or ionic is debatable. At an introductory level showing it as either covalent or ionic is acceptable.
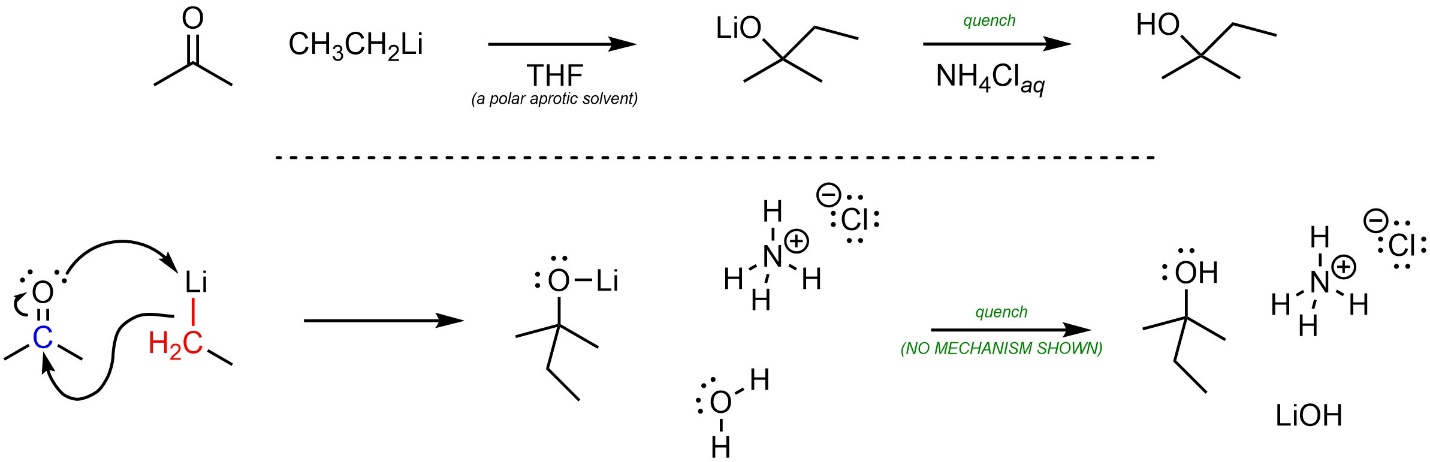
Scheme 7.17 – Approximated Mechanism for Nucleophilic Attack of Ethylmagnesium Bromide onto Acetone.
Again, it is important to remember that this is an approximation. Some sources may show other, more complex mechanisms for these reactions.

