7.2. Terminology: Nucleophiles and Electrophiles
The following new terms form the basis for most chemical reactions at an introductory level. It is vital that these concepts be understood for successful understanding of the rest of the text.
Electrophiles (“electron-lovers”): An electrophile is a group (or atom) that wants to accept electrons. Often this is the result of having a deficiency of electron density on the group/atom from a permanent dipole and/or partial positive (cationic) charge due to resonance delocalization.
Carbonyls are electrophiles (see Figure 7.1). To avoid ambiguity, usually you refer to the atom(s) that are electron-deficient as being electrophilic. For example, one would say that the carbon atom of the carbonyl is electrophilic. The oxygen atom is not electrophilic.
Nucleophiles (“nucleus-lovers”): A nucleophile is a group (or atom) that wants to donate electrons. Often this is the result of having an excess of electron density on the group/atom from a permanent dipole and/or partial negative (anionic) charge due to resonance delocalization.
Alcohols are nucleophiles (Figure 7.2). To avoid ambiguity, usually you refer to the atom(s) that are electron-rich as being nucleophilic. For example, one would say that the oxygen atom of the alcohol is nucleophilic. The carbon and hydrogen atoms are not nucleophilic. Alkoxides are even better nucleophiles because they have even more electron density on the nucleophilic atom.
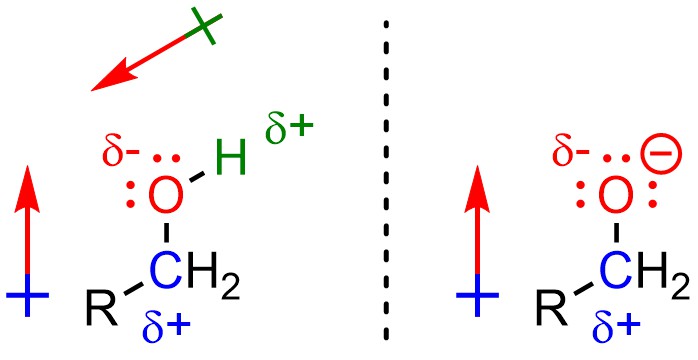
Figure 7.2 – Alcohols and Alkoxides as Nucleophiles.
Technically carbonyls are also nucleophiles (see Figure 7.1); the oxygen atom in the carbonyl is nucleophilic. However, most reactions where carbonyls behave as nucleophiles are more advanced, with lengthy and/or complicated mechanisms.
It can be challenging to determine if an atom is electrophilic or nucleophilic (or neither) when charged species are being analyzed. For example, despite having a cationic charge the nitrogen of an iminium is not electrophilic (Figure 7.3).
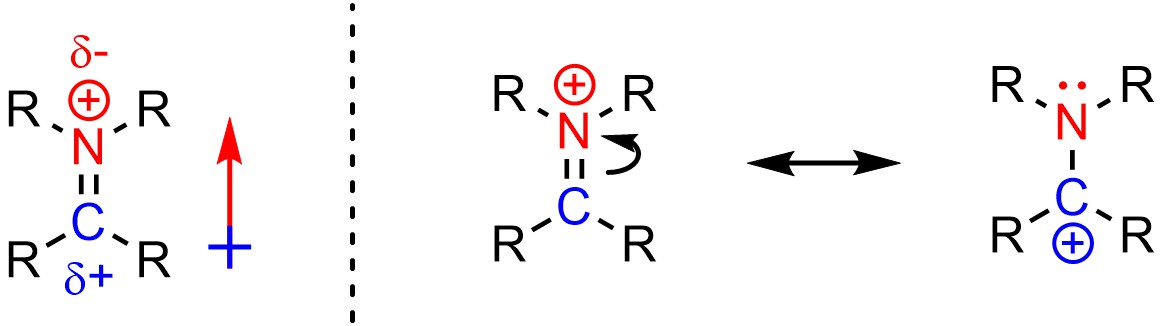
Figure 7.3 – Electrophilicity and Iminium Cations.
This can be rationalized in two ways. First, because nitrogen is more electronegative than carbon the bond between them is polar, with a partial positive on the carbon atom and a partial negative on the nitrogen atom. Second, the nitrogen atom is not able to easily accept additional electron density because it already has a full octet in both resonance forms. Conversely, the carbon atom of an iminium is an electrophile because it has a partial positive from the dipole and has an incomplete octet in one of the resonance forms.
The same arguments may be applied to other compounds, such as hydronium (Figure 7.4): the dipole and full octet mean that, despite a cationic charge, the oxygen atom is not electrophilic. Conversely, the hydrogen atoms are. This demonstrates and important difference between formal and partial charges.
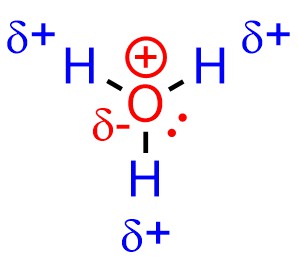
Figure 7.4 – Electrophilicity and Hydronium Cations.
Anionic examples may also be challenging. Despite having a negative charge the boron atom of borohydride is not nucleophilic (Figure 7.5).
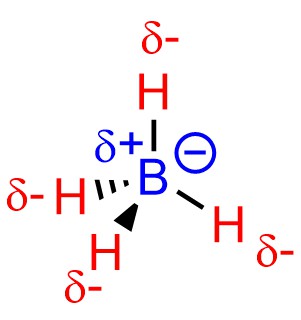
Figure 7.5 – Nucleophilicity and Borohydride Anions.
When describing nucleophile-electrophile reactions the most common phrasing is to say that the nucleophile “attacks” the electrophile, with “attacks” representing the donation of electrons from one to the other. In all nucleophile-electrophile reactions the nucleophile forms a new bond by sharing a pair of electrons with an electrophile. Reactions where the nucleophile and electrophile combine to form a new, larger molecule are sometimes referred to as addition reactions.
It is important when proposing nucleophile-electrophile reactions to avoid violating the octet rule. For example, if a nucleophile is attacking an electrophile that already has a full octet, the electrophile will have to lose electrons to avoid having more than a full valence. Most often this is done by breaking another bond at the same time the new bond is being formed.
7.2.1. Relationship to Acid-Base Reactions
The definitions of electrophile and nucleophile are functionally identical to the definitions of Lewis acids and Lewis bases, respectively. The distinction of terms is semi-arbitrary, though most organic chemists reserve Lewis acid-base naming for reactions where the Lewis acid (electrophile) is a metal or metalloid. This text will focus on the use of nucleophile/electrophile descriptors in place of Lewis base/acid.
Technically, under the definitions of nucleophile and electrophile all Brønsted acid-base reactions are also nucleophile-electrophile reactions (Figure 7.6). The proton of the acid is electron-deficient and can be classed as an electrophile, while the base is electron-rich and can be classed as a nucleophile.
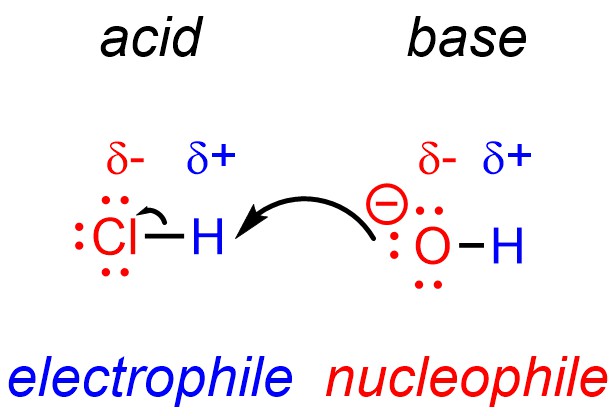
Figure 7.6 – Acid-Base Reactions as Nucleophile-Electrophile Reactions.
Acid-base reactions are simply a common type of nucleophile-electrophile reaction. However, there are often (extreme) differences in the rates of reactions between Brønsted acid-base reactions and other nucleophile-electrophile reactions, with acid-base reactions generally being much faster. As a result, the distinction is still made between the two reactivities.
7.2.2. Comparing Nucleophiles
At an introductory level nucleophilicity and basicity may be considered equivalent: whichever compound is a stronger base is also a stronger nucleophile. However, it is important to point out that this is technically inaccurate. Some compounds are strong bases but weak or moderate nucleophiles, and vice versa. This results from several related/competing factors, and an in-depth discussion is best left for more advanced texts. As such, this text will clearly indicate if a molecule is nucleophilic without being basic (or vice versa).
7.2.3. Comparing Electrophiles
Comparing two or more electrophiles requires a comparison of steric and electronic parameters.
For example, aldehydes are more electrophilic than ketones. Aldehydes have one large group and a hydrogen, while ketones have two large groups (Figure 7.7). Even the smallest alkyl group, CH3, is much larger than H. As a result, there are fewer steric interactions felt by the nucleophile as it attacks an aldehyde compared to a ketone. It is easier to attack an aldehyde than a ketone because there is less steric strain in the transition state. The aldehyde is a better electrophile sterically.
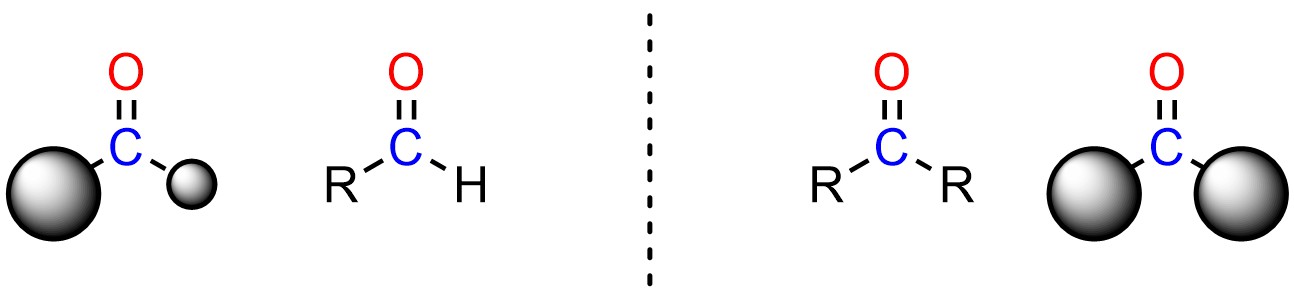
Figure 7.7 – Comparison of Steric Differences Between Aldehydes and Ketones.
When comparing electronic factors consider the same factors that stabilized/destabilized anionic and cationic charges (see Section 6.3). Any factor that removes electron density from (destabilizes) the electrophile will increase electrophilicity, while any factor that increases electron density on (stabilizes) the electrophile will decrease electrophilicity.
To continue the above example, recall that alkyl groups stabilize adjacent cations (see Section 6.3.6.). The resonance form of a ketone with a carbocation has two stabilizing alkyl groups, while the equivalent resonance form of the aldehyde only has one (Figure 7.8). As a result, the partial positive charge on the carbon of a ketone is more stable (smaller) than the equivalent charge in an aldehyde. Since it is more stable (lower in energy) it requires more energy to be attacked by a nucleophile. It is easier to attack an aldehyde than a ketone because it is more electron deficient. The aldehyde is a better electrophile electronically.
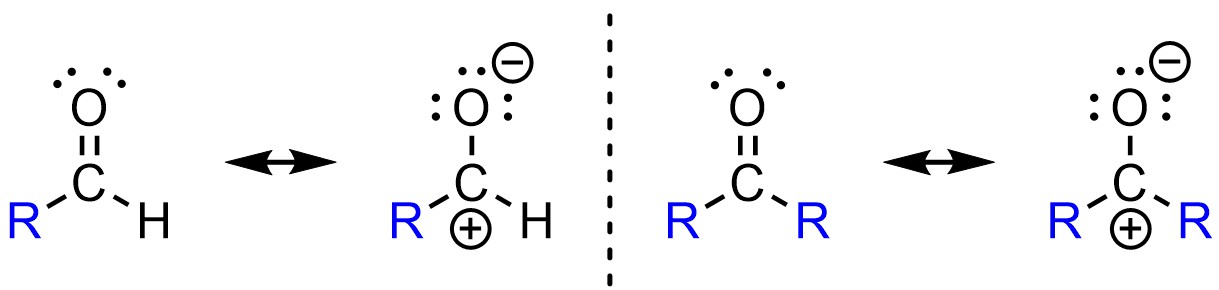
Figure 7.8 – Comparison of Electronic Differences Between Aldehydes and Ketones.
The electronic difference between ketones and aldehydes is small. However, the electronic difference between ketones and esters is very large (Figure 7.9). Functional groups with additional resonance stabilization of the electrophilic site are much more stable than those without. Since it is more stable (lower in energy) it requires more energy to be attacked. It is much easier to attack a ketone than an ester because it has more electron deficiency at the electrophilic atom. The ketone is a much better electrophile electronically.
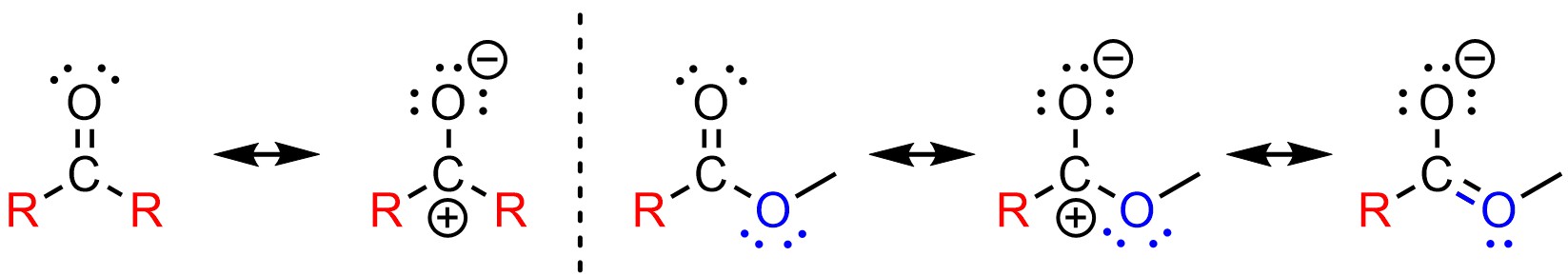
Figure 7.9 – Comparison of Electronic Differences Between Ketones and Esters.

