Chapter 5. Minerals
Adapted by Karla Panchuk from Physical Geology by Steven Earle

Learning Objectives
After reading this chapter and answering the review questions at the end, you should be able to:
- List the criteria required for a substance to be considered a mineral.
- Explain how atoms bond within minerals.
- Explain how mineral lattices influence the properties of minerals.
- Summarize the categories of minerals defined by anions or anionic groups.
- Describe the types of configurations of silica tetrahedra found in silicate minerals.
- Explain how minerals form.
- Describe the key properties for identifying minerals.
What Is a Mineral?
Minerals are all around us: the graphite in your pencil, the salt on your table, the plaster on your walls, and the trace amounts of gold in your computer. Minerals can be found in a wide variety of consumer products such as paper, medicine, processed foods, and cosmetics. And of course, everything made of metal is also derived from minerals.
A mineral is a naturally occurring solid made of specific elements, and arranged in a particular repeating three-dimensional structure.
“Naturally occurring” means that minerals can be formed from substances and under conditions found in nature. Substances that can only be made by humans—classified as anthropogenic materials—do not count as minerals, nor do substances produced by natural processes acting upon anthropogenic materials.
In the context of the definition of minerals, “solid” means solid at 25ºC. There are some exceptions to this rule, made for substances defined as minerals before 1959, prior to strict procedures being established for determining what is or isn’t a mineral. One example is ice, which is only solid at or below 0 °C. Another is mercury, which is solid below -39 ºC. Mercury that is present in rocks at temperatures above -39 ºC appears as silvery blobs of liquid (Figure 5.2).

“Specific elements” means that minerals have a specific chemical formula or composition. The mineral pyrite, for example, is FeS2 (two atoms of sulphur for each atom of iron), and any significant departure from that formula would make it a different mineral. Some minerals can have variable compositions within a specific range. The mineral olivine, for example, has a formula written as (Fe,Mg)2SiO4, because the composition of olivine can range all the way from Fe2SiO4 to Mg2SiO4, and have any proportion of iron and magnesium in between. This type of substitution is known as solid solution.
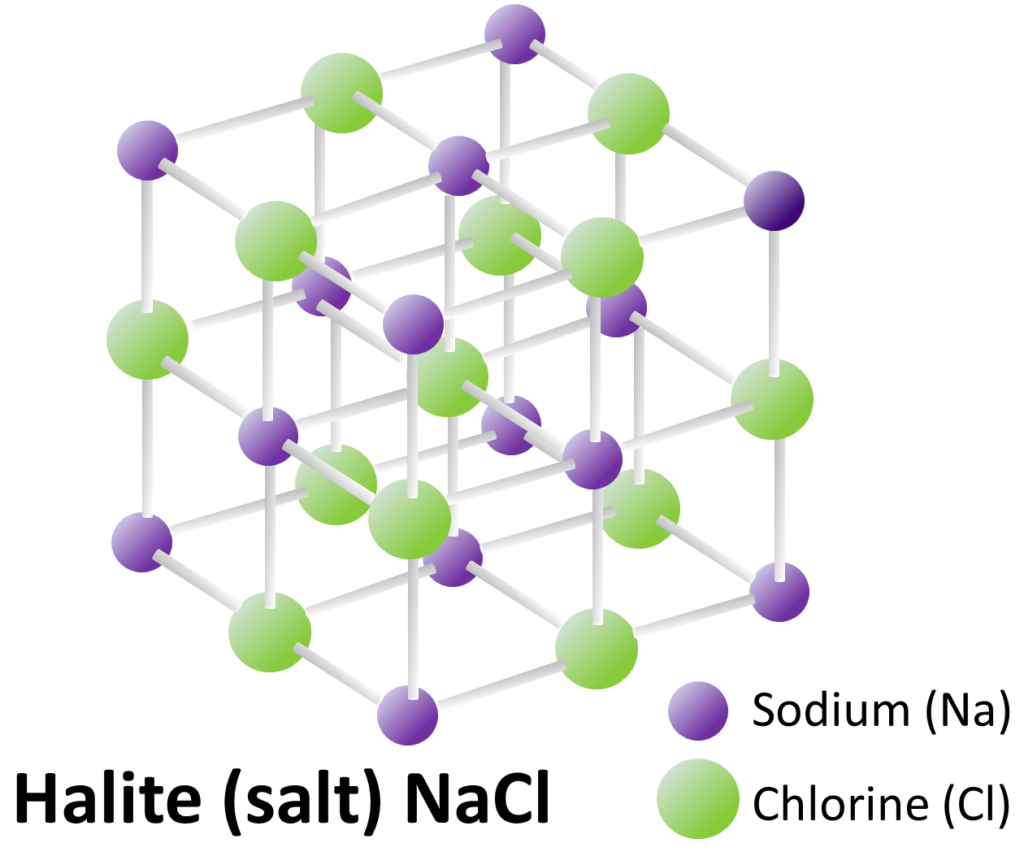
Most important of all, the atoms within a mineral are arranged in a specific repeating three-dimensional structure or lattice. This regular structure means that all minerals are crystals. The mineral halite, which we use as table salt, has a relatively simple crystal lattice (Figure 5.3). Atoms of sodium (Na, purple) alternate with atoms of chlorine (Cl, green). The chemical bonds holding the Na and Cl atoms together are all at 90º to each other. Even tiny crystals, like the ones in your salt shaker, have lattices that extend in three dimensions for thousands of repetitions. Halite will always have this structure, and will always have the formula NaCl.
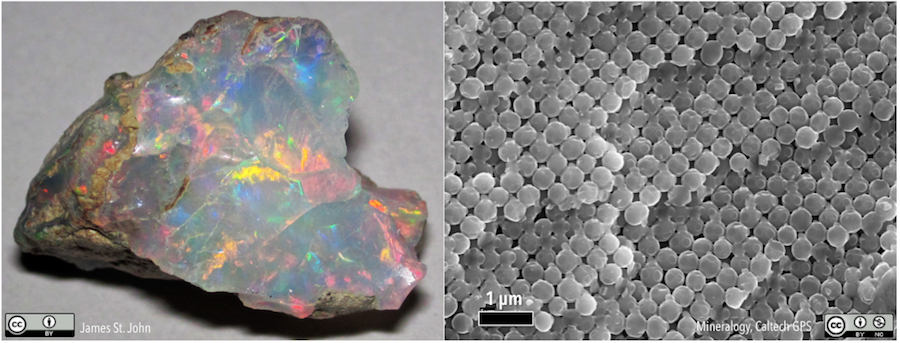
Some mineral-like materials do not have a regular internal atomic arrangement. Opal (Figure 5.4) is one example. In many respects it fits the definition of a mineral: it has a specific chemical composition (SiO2·nH2O, where n means that there can be varying amounts of water in the structure), forms naturally through geological processes, and is solid at 25 ºC. However, the structure of opal consists of closely packed spheres (Figure 5.4, right) rather than a lattice like halite. Substances like opal, which are mineral-like, but which do not have a crystalline structure, are called mineraloids.
References
Nickel, E. H. (1995). The Definition of a Mineral. The Canadian Mineralogist 33, 698-690. Read paper
Williams, P. (2010, July 28). Deadliest place on Earth? Surviving Cueva de los Cristales – The Giant Crystal Cave. Visit website

