5.1 Atoms
Protons Are What Make Elements Distinct
All matter, including mineral crystals, is made up of atoms. All atoms are made up of three main particles: protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. Protons and neutrons have approximately the same mass, but electrons have a mass that is 10,000 times smaller.
The element hydrogen (H) has the simplest atoms. Most hydrogen atoms have just one proton and one electron. The proton forms the nucleus (the centre of the atom), while the electron orbits around it (Figure 5.5, left). All other elements have more than one proton in their nucleus. Protons repel each other because they are positively charged, but it is possible to have more than one proton in a nucleus because neutrons hold them together. The next most complex atom, helium (He) has two protons and two neutrons in its nucleus, in its most common form. Some atoms of the same element can have different numbers of neutrons. For example, forms of hydrogen exist with one and two neutrons, and a tiny fraction of He atoms have only one neutron. Forms of an element with different numbers of neutrons are called isotopes.
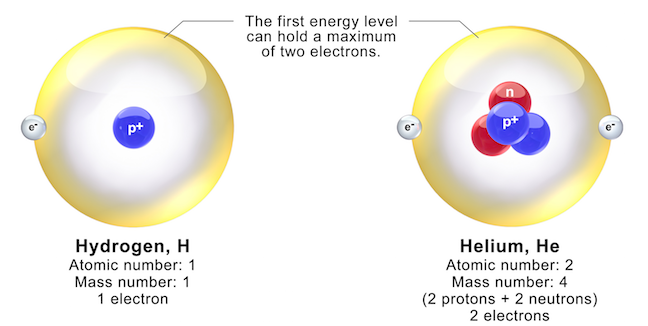
The number of protons in an atom determines what element it will be, so the number of protons is called the atomic number of that element. The total number of protons and neutrons in the nucleus is the mass number. The mass number distinguishes between isotopes of an element. Isotopes of an element are denoted by putting the mass number as a subscript in front of the symbol for that element. For example, the isotopes of hydrogen are 1H (1 proton), 2H (1 proton + 1 neutron), and 3H (1 proton + 2 neutrons).
For most of the 16 lightest elements (up to oxygen) the number of neutrons is equal to the number of protons. For most of the remaining elements, there are more neutrons than protons. This is because the more protons that are concentrated in a small space, the more neutrons are needed to keep the nucleus together. The most common isotope of uranium (U), for example, is 238U. It has 92 protons, but requires 146 neutrons to keep them together. The neutrons are only partly successful. Uranium is radioactive, meaning that its nucleus will eventually split apart and release energy. What remains of the nucleus has fewer protons, so after decay the atom is a different element.
Electrons Are What Control How Atoms Interact
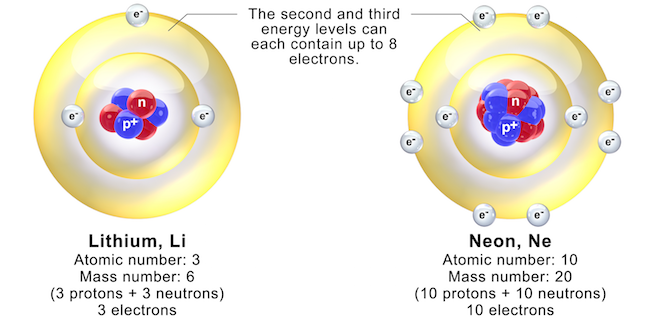
Electrons orbiting around the nucleus of an atom are arranged in shells (also called energy levels). The first shell can hold only two electrons (as in H and He in Figure 5.5), but the next shell holds up to eight electrons. An atom can have many shells of electrons, but there are never more than 8 outermost electrons interacting with surrounding atoms.
The outermost electrons determine how atoms can be bonded together. Elements that have a full outer shell (e.g., neon, Figure 5.6 right) are inert because they do not react with other elements to form compounds. These are the noble gases (including helium, argon, krypton, and radon, in addition to neon) in the far-right column of the periodic table. For elements that do not have a full outer shell (e.g., lithium, Figure 5.6 left), the outermost electrons can interact with the outermost electrons of nearby atoms to create chemical bonds.
The electron shell configurations for 29 of the first 36 elements are listed in Table 5.1. Note that some of the shells in the table below have more than 8 electrons. This is because they contain subshells. For example, the third shell can hold up to 18 electrons because it contains one subshell that can hold 2 electrons, and two subshells that can hold 8 electrons each.
| Table 5.1 Electron shell configurations of some of the elements up to krypton. Inert elements (those with filled outer shells) are shaded. | ||||||
| Number of Electrons in Each Shell | ||||||
| Element | Symbol | Atomic Number | First | Second | Third | Fourth |
| Hydrogen | H | 1 | 1 | |||
| Helium | He | 2 | 2 | |||
| Lithium | Li | 3 | 2 | 1 | ||
| Beryllium | Be | 4 | 2 | 2 | ||
| Boron | B | 5 | 2 | 3 | ||
| Carbon | C | 6 | 2 | 4 | ||
| Nitrogen | N | 7 | 2 | 5 | ||
| Oxygen | O | 8 | 2 | 6 | ||
| Fluorine | F | 9 | 2 | 7 | ||
| Neon | Ne | 10 | 2 | 8 | ||
| Sodium | Na | 11 | 2 | 8 | 1 | |
| Magnesium | Mg | 12 | 2 | 8 | 2 | |
| Aluminum | Al | 13 | 2 | 8 | 3 | |
| Silicon | Si | 14 | 2 | 8 | 4 | |
| Phosphorus | P | 15 | 2 | 8 | 5 | |
| Sulphur | S | 16 | 2 | 8 | 6 | |
| Chlorine | Cl | 17 | 2 | 8 | 7 | |
| Argon | Ar | 18 | 2 | 8 | 8 | |
| Potassium | K | 19 | 2 | 8 | 8 | 1 |
| Calcium | Ca | 20 | 2 | 8 | 8 | 2 |
| Scandium | Sc | 21 | 2 | 8 | 9 | 2 |
| Titanium | Ti | 22 | 2 | 8 | 10 | 2 |
| Vanadium | V | 23 | 2 | 8 | 11 | 2 |
| Chromium | Cr | 24 | 2 | 8 | 13 | 1 |
| Manganese | Mn | 25 | 2 | 8 | 13 | 2 |
| Iron | Fe | 26 | 2 | 8 | 14 | 2 |
| . | . | . | . | . | . | . |
| Selenium | Se | 34 | 2 | 8 | 18 | 6 |
| Bromine | Br | 35 | 2 | 8 | 18 | 7 |
| Krypton | Kr | 36 | 2 | 8 | 18 | 8 |

