10.1 Controls on Metamorphic Processes
The main factors that control metamorphic processes are:
- The chemical composition of the parent rock
- The temperature at which metamorphism takes place
- The pressure applied, and whether the pressure is equal in all directions or not
- The amount and type of fluid (mostly water) that is present during metamorphism
- The amount of time over which metamorphic conditions are sustained
Mineral composition
Parent rocks can be from any of the three rock types: sedimentary, igneous, or metamorphic. The critical feature of the parent rock is its mineral composition. This is because the stability of minerals—how they are influenced by changing conditions—is what determines which minerals form as metamorphism takes place. When a rock is subjected to increased temperatures and pressures, some minerals will undergo chemical reactions and turn into new minerals, while others might just change their size and shape.
Temperature
The temperature under which metamorphism occurs is a key variable in determining which metamorphic reactions happen. Mineral stability depends on temperature, pressure, and the presence of fluids. Minerals are stable over a specific range of temperatures. Quartz, for example, is stable from surface temperatures up to approximately 1800°C. If the pressure is higher, that upper limit will also be higher. If there is water present, it will be lower. Most other common minerals have upper limits between 150°C and 1000°C.
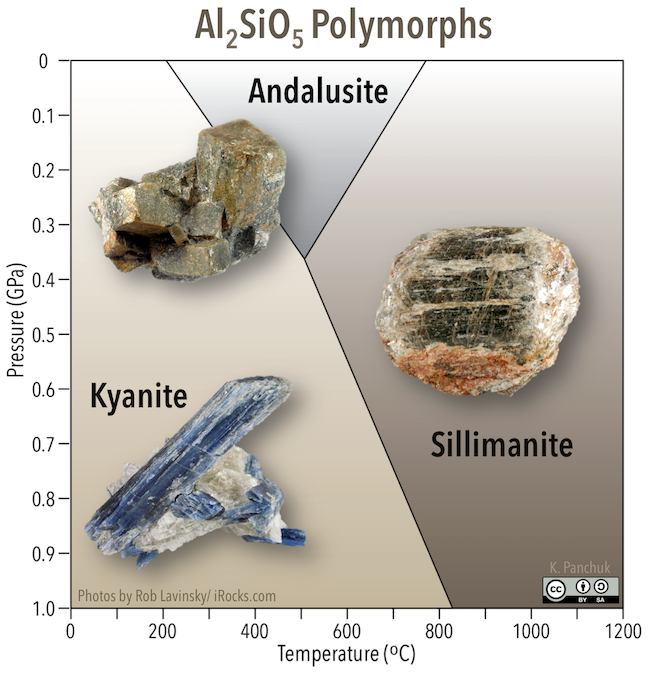
Some minerals will change their crystal structure depending on the temperature and pressure. Quartz has different polymorphs that are stable between 0°C and 1800°C. The minerals kyanite, andalusite, and sillimanite are polymorphs with the composition Al2SiO5. The fact that they are stable at different pressures and temperatures means that their presence can be used to determine the pressures and temperatures experienced by a metamorphic rock that contains one or more of the polymorphs (Figure 10.3).
Pressure
Pressure has implications for mineral stability, and therefore the mineral content of metamorphic rocks, but it also determines the texture of metamorphic rocks. When directed pressure (or directed stress) acts on a rock, it means the stress on the rock is much greater in one direction than another. In an experiment with cylinders of modeling clay stacked in a block (Figure 10.4, top), pushing down on the clay from above resulted in higher directed pressure in the up-down direction (larger arrows; downward from pushing on the clay, and upward from the force of the table beneath the clay) than in the sideways direction, where only air pressure was acting (small arrows). The clay cylinders became elongated in the direction of least pressure.
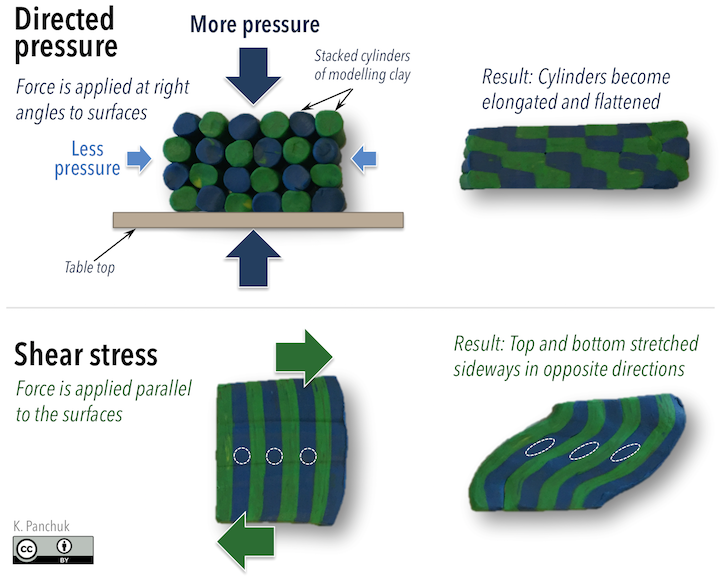
Rocks undergo shear stress when forces act parallel to surfaces. In another modelling-clay experiment, applying oppositely directed forces to the top and bottom of a block of clay (Figure 10.4, bottom) caused diagonal stretching within the block. Note the change in shape of the dashed white reference circles.
In both experiments, parts of the clay became elongated in a particular direction. When mineral grains within a rock become aligned like this, it produces a fabric called foliation. Foliation is described in more detail later in this chapter.
Fluids
Water is the main fluid present within rocks of the crust, and the only one considered here. The presence of water is important for two main reasons. First, water facilitates the transfer of ions between minerals and within minerals, and therefore increases the rates at which metamorphic reactions take place. This speeds the process up so metamorphism might occur more rapidly, or metamorphic processes that might not otherwise have had time to be completed are completed.
Secondly, water—especially hot water—can have elevated concentrations of dissolved substances, making it an important medium for moving ions from one place to another within the crust. Processes facilitated by hot water are called hydrothermal processes (hydro refers to water, and thermal refers to heat).
Time
Most metamorphic reactions occur very slowly. Estimates of the growth rates of new minerals within a rock during metamorphism suggest that new material is added to the outside of mineral crystals at a rate of approximately 1 mm per million years. Very slow reaction rates make it difficult to study metamorphic processes in a lab.
While the rate of metamorphism is slow, the tectonic processes that lead to metamorphism are also very slow, so there is a good chance that metamorphic reactions will be completed. For example, an important setting for metamorphism is many kilometres deep within the roots of mountain ranges. A mountain range takes tens of millions of years to form, and tens of millions of years more to be eroded to the extent that we can see the rocks that were metamorphosed deep beneath it.
Exercise: How Long Did It Take?
The large reddish crystals in Figure 10.5 are garnet, and the surrounding light coloured rock is dominated by muscovite mica. The Euro coin is 23 mm in diameter. Assume that the diameters of the garnets increased at a rate of 1 mm per million years. Based on the approximate average diameter of the garnets visible, estimate how long this metamorphic process might have taken.


