7.1 Magma and How It Forms
Igneous rocks form when melted rock cools. Melted rock originates within Earth as magma. Magma compositions vary, but will have eight main elements in different proportions. The most abundant elements are oxygen and silicon, followed by aluminum, iron, calcium, sodium, magnesium, and potassium. These eight elements are also the most abundant in Earth’s crust (Figure 7.2). All magmas have varying proportions of lighter elements such as hydrogen, carbon, and sulphur. Lighter elements are converted into gases like water vapour, carbon dioxide, hydrogen sulphide, and sulphur dioxide as the magma cools.
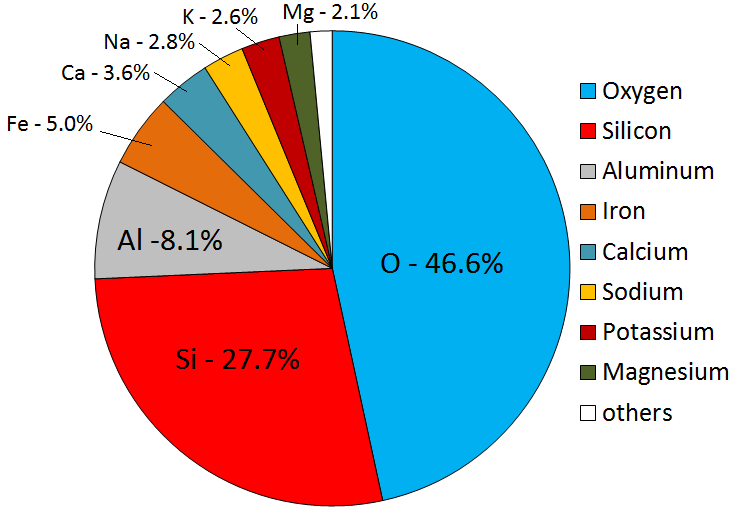
Magma composition depends on the composition of the rocks that melted to form the magma, and on the conditions under which the melting happened. Most igneous rock in Earth’s crust comes from magmas that formed through partial melting of existing rock, either in the upper mantle or the crust. During partial melting, only some of the minerals within a rock melt. This happens because different minerals have different melting temperatures. The melt is less dense than the surrounding rock, and will percolate upward without the source rock having melted completely. The result is magma with a different composition than the original rock. Partial melting produces melt that has more silica than the original rock, because minerals higher in silica have lower melting points.
To see how partial melting works, consider the mix of materials in Figure 7.3a. It contains white blocks of candle wax, black plastic pipe, green beach glass, and pieces of aluminum wire. When the mixture is heated to 50 °C in a warm oven, the wax melts into a clear liquid (Figure 7.3b), but the other materials remain solid. This is partial melting.

When the mixture is heated to 120 °C, the plastic melts and mixes with the wax, but the aluminum and glass still remain solid (Figure 7.3c). This is still considered partial melting because solid materials remain. When the plastic and wax mixture is poured into a separate container and allowed to cool, the resulting solid has a very different composition from the original mixture (Figure 7.3d). The plastic and wax are analogous to more silica-rich minerals with relatively lower melting points than other minerals in the same rock.
Of course, partial melting in the real world isn’t as simple as the example in Figure 7.3. Many rocks are much more complex than the four-component system used here. Some mineral components of rocks may have similar melting temperatures, and begin to melt at the same time. The melting temperature of a mineral may change in the presence of other minerals. Also, when rocks melt, the process can take millions of years, unlike the 90 minutes required to melt the pipe and wax in the experiment in Figure 7.3.
Why Rocks Melt
The magma that is produced by partial melting is less dense than the surrounding rock. Magma from partial melting of mantle rocks rises upward through the mantle, and may pool at the base of the crust, or rise through the crust. Moving magma carries heat with it, and some of that heat is transferred to surrounding rocks. If the melting temperature of a rock is less than the temperature of the magma, the rock will begin to melt, and the composition of the magma may change to reflect a mixture of sources. But adding heat is not the only way to trigger melting.
Decompression Melting
Earth’s mantle is almost entirely solid rock, in spite of temperatures that would cause rock at Earth’s surface to melt. Mantle rock remains solid at those temperatures because the rock is under high pressure. This means that melting can be triggered without adding heat if the rock is already hot enough, and the pressure is reduced (Figure 7.4, left, white dashed boxes). Melting triggered by a reduction in pressure is called decompression melting.

Pressure is reduced when mantle rocks move upward due to convection, or rise as a plume within the mantle. Pressure is also reduced where the crust thins, such as along rift zones.
Flux-induced Melting
When a substance such as water is added to hot rocks, the melting points of the minerals within those rocks decreases. If a rock is already close to its melting point, the effect of adding water can be enough to trigger partial melting. The added water is a flux, and this type of melting is called flux-induced melting. In Figure 7.4 (right), the rock (represented by the dashed box) is not hot enough to be right of the line where dry mantle rocks melt, but it is to the right of the line where wet mantle rocks melt.
Flux-induced partial melting of rock happens in subduction zones. Minerals are transformed by chemical reactions under high pressures and temperatures, and a by-product of those transformations is water. Relatively little water is required to trigger partial melting. In laboratory studies of the conditions of partial melting in the Japanese volcanic arc, rocks with only 0.2% of their weight consisting of water melted by up to 25%.
Cooling Magma Becomes More Viscous
Viscosity refers to the ease with which a substance flows. A substance with low viscosity is runnier than a substance with high viscosity. At temperatures over 1300°C, most magma is entirely liquid because there is too much energy for the atoms to bond together. As magma loses heat to the surrounding rocks and its temperature drops, things start to change. Silicon and oxygen combine to form silica tetrahedra. With further cooling, the tetrahedra start to link together into chains, or polymerize. These silica chains make the magma more viscous. Magma viscosity has important implications for the characteristics of volcanic eruptions.
Exercise: Making Magma Viscous
This is a quick and easy experiment that you can do at home to help you understand the properties of magma. It will only take about 15 minutes, and all you need is half a cup of water and a few tablespoons of flour.
If you’ve ever made gravy, white sauce, or roux, you’ll know how this works.
Place 1/2 cup (125 mL) of water in a saucepan over medium heat. Add 2 teaspoons (10 mL) of white flour and stir while continuing to heat the mixture until boiling. The white flour represents silica. The mixture should thicken like gravy because the gluten in the flour becomes polymerized into chains during this process.
Now add more “silica” to see how this changes the viscosity of your magma: take another 4 teaspoons (20 mL) of flour and mix it thoroughly with 4 teaspoons (20 mL) of water in a cup. Add that mixture to the rest of the water and flour in the saucepan. Stir while bringing it back up to nearly boiling temperature, and then allow it to cool. This mixture should slowly become much thicker (Figure 7.5) because there is more gluten, and more chains have formed.

References
Kushiro, I. (2007). Origins of magmas in subduction zones: a review of experimental studies. Proceedings of the Japan Academy, Series B, Physical and Biological Sciences 83(1), 1-15. Read the paper

