Appendix I. Chemistry
It is helpful to review some key concepts from high school chemistry to prepare for this course. These are concepts that are generally covered in schools in Saskatchewan in Grade 10 science class (or earlier). This appendix contains outcomes, vocabulary, and review questions in chemistry.
Note that this appendix is not intended to re-teach you high school chemistry; it provides guidance on aspects of high school chemistry that will be useful as background for this introductory physical geology course. If you are struggling with these concepts please approach your instructor to chat about other resources to help you.
AI-1. Outcomes
After reviewing this appendix the learner should be:
- familiar with the structure and content of the periodic table,
- able to write the one or two letter codes for key elements such as iron and oxygen,
- familiar with key vocabulary for physical and chemical processes, and be able to use these words in sentences and provide examples of their importance in their everyday life,
- be prepared to give examples of common items that have acid, neutral, and basic pH.
AI-2. Vocabulary
Review this list of vocabulary. Try to define each one out loud, and create a sentence using each word in context. You can look them up on Wikipedia if you need help remembering their meaning.
- periodic table
- element
- atomic weight
- atomic mass
- atomic number
- atom
- matter
- molecule
- solid
- liquid
- gas
- conditions
- acid
- base
- react
- charge
- gain
- lose
- repel
- attract
- pH
- heat
- cold
- melt
- boil
- freeze
- melting point
- boiling point
- freezing point
- solidify
- metal
- noble gas
- halide
- alkali metal
- transition element
- chemical bond
- inert
- density
- mass
- heavy
- light
- variations
- chemical reaction
- chemical properties
- chemical formula
- composition
- increase
- decrease
- water
- ice
- seawater
- salt
- substance
- freshwater
- dissolve
- solute
- solvent
- dilute
- concentrate
- evaporate
- condense
- precipitate / precipitation
- component
- mixture
- transformation
- stable
- unstable
AI-3. Review Questions
Work your way through the following review questions. If you are not able to answer the questions on your own, look the terms in the question up on Wikipedia to get more background information to help you answer the question.
- Describe the differences between a solid, a liquid, and a gas. Give an example of each from your daily life.
- What happens to liquid water if you cool it to below 0˚C? Note: this depends on atmospheric pressure, but for the purposes of this question you can consider what would happen if you did this in Saskatoon.
- What happens to liquid water if you heat it to greater than 100˚C? Note: this depends on atmospheric pressure, but for the purposes of this question you can consider what would happen if you did this in Saskatoon.
- What happens if water vapour (e.g., from a boiling kettle) hits a cold mirror?
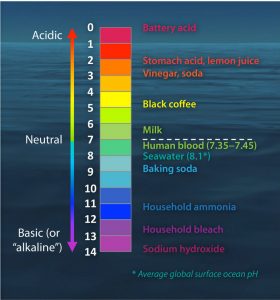
- What is a neutral pH? What is an acidic pH? What is a basic pH?
- Give examples of food or household items that are acids and bases (you can search on google if you don’t know any off the top of your head, or refer to figure AI-2 below).
- When you put an egg into a cola drink, the shell will dissolve (you can try it if you haven’t before!). Is the cola and acid or a base?
- What is the difference between an atom and a molecule?
- Is water an atom or a molecule?
Exploring the periodic table
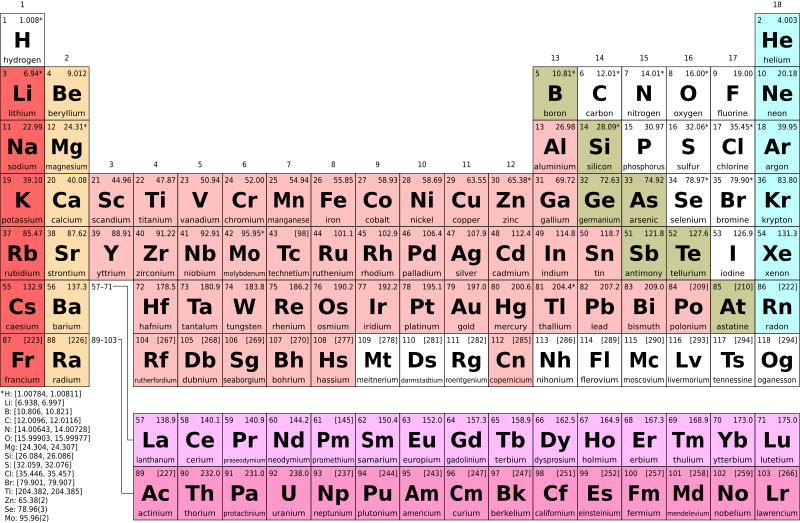
- Examine the periodic table in figure AI-1 (located at the start of this appendix) and note the following:
- Each box in the table represent one of the elements.
- Each element has a set of one or two letters that are used as a code to represent that element. For example, the element gold is represented by the letters “Au”.
- Some of the letter codes for elements do not seem intuitive, but there is a reason for each one. The letter abbreviations are often from the Latin names for the elements. For example, gold in Latin is Aurum.
- What defines an element is the number of protons that element contains. Each element has a specific number of protons. The number of neutrons in each atom of an element can vary, but the number of protons stays the same (or if a proton is lost, the atom becomes a different element).
- The number in the top left corner of each element box is the atomic number for that element – the number of protons in an atom of that element.
- The atomic mass is the sum of the protons plus neutrons for an element. It will vary with different isotopes of the element, since the number of neutrons will vary between isotopes (but not the number of protons, because then they would be different elements!). The units of atomic mass are atomic mass units.
- The number in the top right corner of each element box is the average atomic mass. This is the average mass of an atom of that element. There are different isotopes for most elements and the atomic weight is the weighted average (by abundance) of the masses of these isotopes.
- Note the numbers along the top of the periodic table; these are called groups. The elements in each group generally behave similarly when they bind to other elements.
- The horizontal rows of the periodic table are called periods.
- The colours of the boxes correspond to groups or periods of elements that are grouped by common properties (e.g., the light blue elements are noble gases)
- The video introduction to the periodic table by CrashCourse may also be helpful as you review the periodic table.
- What are some properties that are similar between the elements in each set of boxes with the same colour?
- Figure AI.3 is a periodic table that is missing the names of the elements, but still has the one or two letter codes for each element. Find the following elements in this table. These are elements that will come up in introductory physical geology, so it will help to be familiar with them. You may need to refer to Figure AI-1 to find the elements; if that is the case, review this exercise again until you know the code for that element and can find it without checking the other table.
- iron
- sodium
- magnesium
- chlorine
- aluminum
- hydrogen
- helium
- oxygen
- sulfur / sulphur
- zinc
- lead
- gold
- silver
- uranium
- potassium
- argon
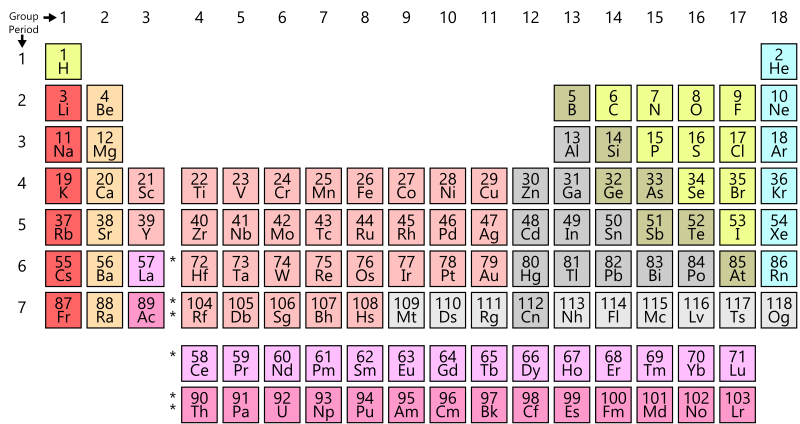
- Look up each element listed in the previous question on Wikipedia.
- How common is each element in nature?
- Is the element generally found as a gas, solid, or liquid?
- Do you encounter each element in the list each day? If you do, where? In what form (e.g., what elements are found in air, water, steel, and jewellery)?
Note the change in atomic weight as you move across the periodic table, and down the periodic table. The density of the elements (g/cm3, mass divided by volume) is related to the atomic weight of the element but also the volume of space the atoms take up. Gases are less dense than solids or liquids, even if the atomic weight of the element is higher.
- Referring to the density version of the interactive periodic table on RSC.org:
- How does the density of lead compare to the density of carbon?
-
- How does the density of iron compare to the density of mercury? Gold?

