Chapter 2. Minerals
Exercises on Minerals
You can download a pdf copy of this lab to print, and order a copy of the lab manual from the bookstore!
Adapted by Lyndsay R. Hauber & Joyce M. McBeth (2018) University of Saskatchewan from Deline B, Harris R & Tefend K. (2015) “Laboratory Manual for Introductory Geology”. First Edition. Chapter 7 “Minerals” by Randa Harris, CC BY-SA 4.0. View source. Last edited: 8 Jan 2020
Note: much of the overview material for this chapter is replicated in this exercise section for your reference as you complete the lab. You will NOT have access to your lab book or notes for the rock and mineral exam!
Name: _______________
NSID and student number: ____________
Date and lab section time: _____________
TAs’ names: _______________ __________________
Your TAs will check that you have completed the questions correctly at the end of the lab period. Please hold on to your lab notes to help you prepare for the rock and mineral quiz and your lab final exam.
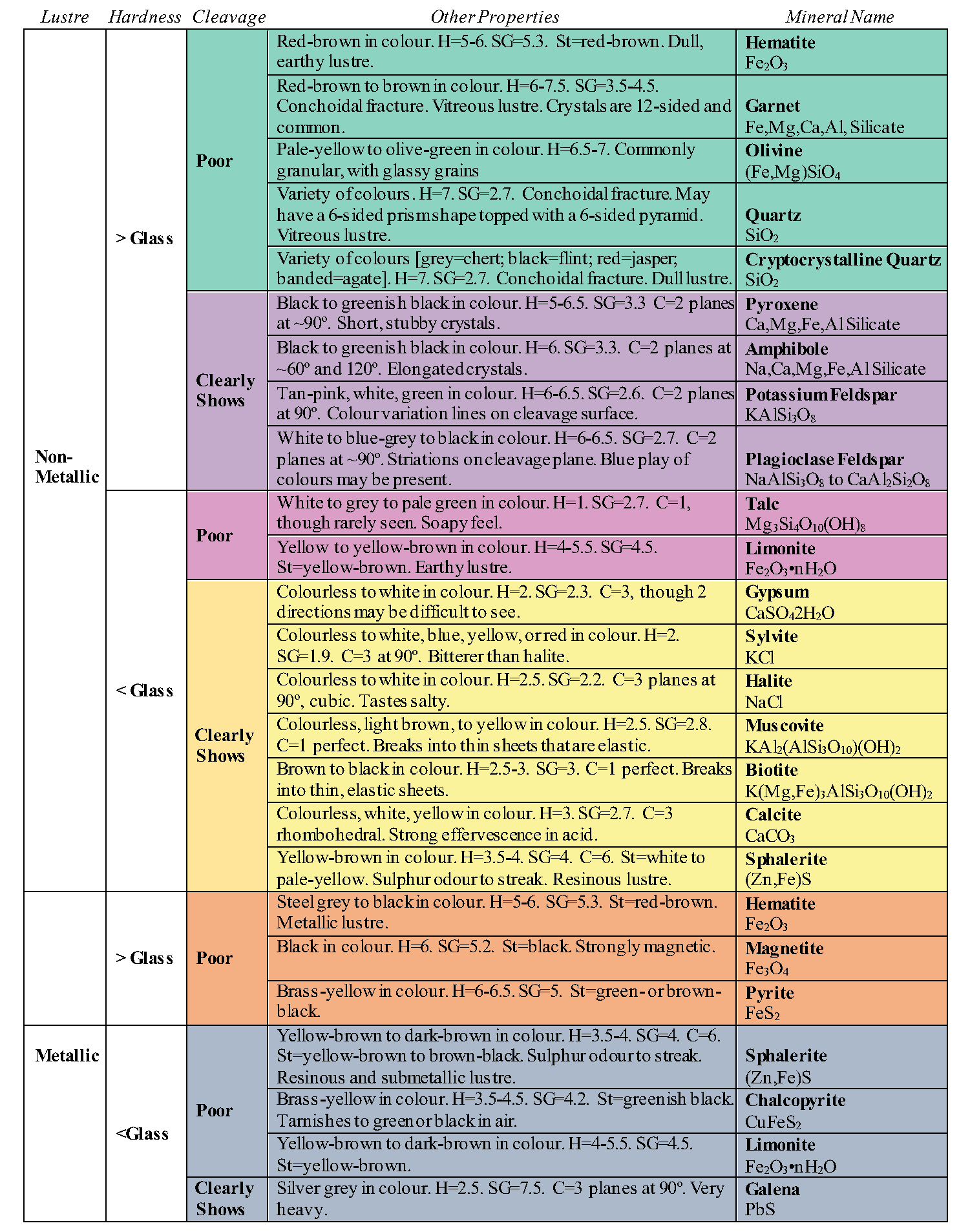
2.2 PHYSICAL PROPERTIES
Identifying a mineral is a little like playing detective. Minerals are identified by their physical properties. How would you describe the mineral in Figure 2.2? You may say that it is shiny, gold, and has a particular shape. Each of these descriptions is a physical property (shiny is lustre, gold is colour, shape is crystal form). Physical properties can vary within the same minerals, so caution should be applied when identifying minerals. For example, colour is a property that is not a very realistic diagnostic tool in many cases, as some minerals, such as Quartz, can come in a variety of colours (e.g. Figure 2.3). Occasionally, colour can be helpful, as in the case of olivine, which is said to be “olive green”, a light to dark green (e.g. Figure 2.4). We will cover each of the physical properties in detail to help you identify the minerals.

Source: Randa Harris (2015) CC BY-SA 3.0 view source

Source: Randa Harris (2015) CC BY-SA 3.0 view source

Source: Joyce M. McBeth (2018) CC BY 4.0 view source
2.2.1 Hardness
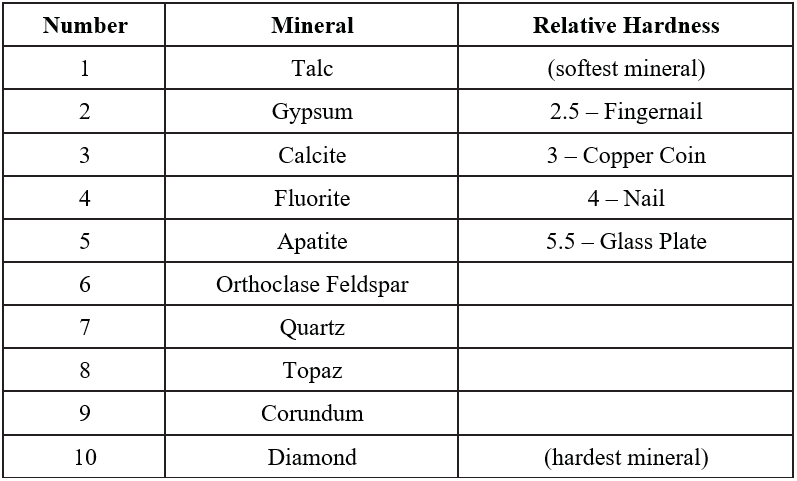
Hardness refers to the resistance of a mineral to being scratched by a different mineral or material and is a product of the strength of the bonds between the atoms of a mineral. Whatever substance does the scratching is harder and the item scratched is softer. Hardness is based off a scale of 1 to 10 created by a mineralogist named Friedrich Mohs (Figure 2.5). Mohs’ scale lists ten minerals in order of relative hardness, with each mineral on the scale able to scratch a mineral of lower number.
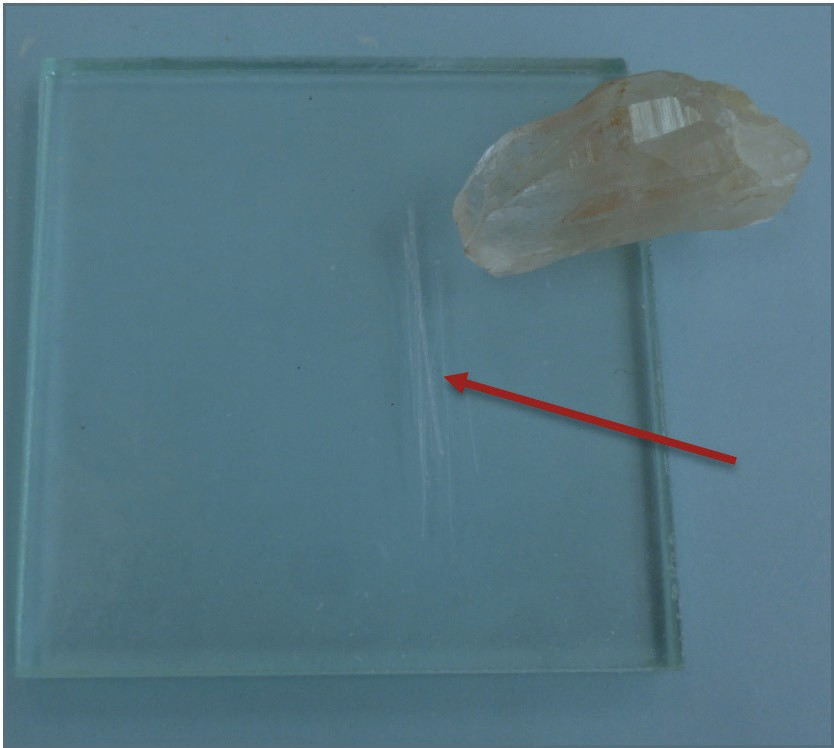
Your mineral kit comes with several items of a known hardness. The glass plate has a hardness of 5.5, the iron nail has a hardness of 4, the copper coin has a hardness of 3, and your fingernail has a hardness of 2.5. If you can scratch a mineral, then it would be softer than your fingernail, so therefore its hardness would be <2.5. When trying to scratch a surface, use force, but be cautious with the glass plate. ALWAYS lay the glass plate on a flat surface rather than holding it in your hand in case it breaks. Do not confuse mineral powder with a scratch – use your finger to feel for a groove created by a scratch (Figure 2.6). in contrast, mineral powder is left behind when a soft mineral scratches a harder surface. Materials of similar hardness have difficulty scratching each other, so that, for example, your fingernail may not be able to always scratch biotite, which has a hardness of 2.5 or gypsum which has a hardness of 2 (Figure 2.7).
Source: Randa Harris (2015) CC BY-SA 3.0 view source
Source: Randa Harris (2015) CC BY-SA 3.0 view source

Source: Randa Harris (2015) CC BY-SA 3.0 view source
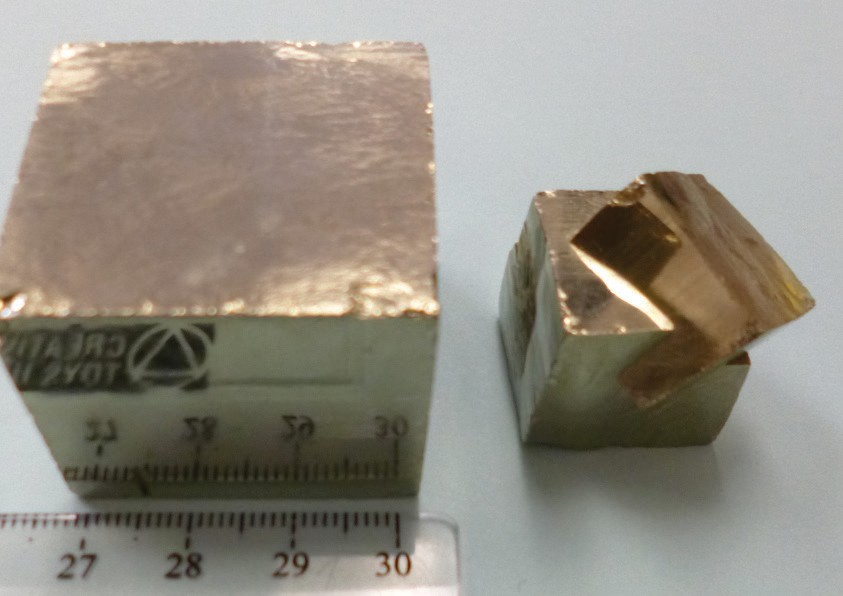
2.2.2 Crystal Form
This property refers to the geometric shape that a crystal naturally grows into and is a reflection of the orderly internal arrangement of atoms within the mineral. If minerals have space to grow when they are developing, they will display their crystal form. These ideal growth conditions do not always occur, however, so many minerals do not display their ideal crystal form due to crowded conditions during growth. Examples of crystal form are shown in Figure 2.8.

Source: Lyndsay Hauber & Joyce M. McBeth (2018) CC BY 4.0, after Randa Harris (2015) CC BY-SA 3.0 view source
2.2.3 Cleavage
As minerals are broken, some may cleave, or break, along smooth flat planes known as cleavage. These flat surfaces are parallel to directions of weakness within the crystal. All the bonds among the atoms within a mineral may not be of the same strength, so that when a mineral is broken, it breaks along these zones of weakness. This results in flat cleavage planes. Minerals with perfect cleavage break along a smooth, flat plane, while those with poor cleavage break in a more irregular fashion. Some minerals do not contain zones of weakness either because all of the bonds are the same strength or the weaker bonds are not aligned within a plane. If this is the case it will not have cleavage; instead, it will fracture, similar to the curved fracture of glass when you get crack in a windshield.
Be careful not to confuse cleavage with crystal form. Crystal form occurs as a mineral grows (e.g., cubes of pyrite), while cleavage only forms as a mineral breaks. See Figure 2.9 for the main types of cleavage and an example of each.

Source: Lyndsay Hauber & Joyce M. McBeth (2018) CC BY 4.0, after Randa Harris (2015) CC BY-SA 3.0 view source

Source: Joyce M. McBeth (2018) CC BY 4.0, after Randa Harris (2015) CC BY-SA 3.0 view source
2.2.4 Fracture
When minerals do not break along cleavage planes, but rather break irregularly, they are said to fracture. Commonly, fracture surfaces are either uneven or conchoidal, a ribbed, smoothly curved surface similar to broken glass (e.g. Figure 2.11).

Source: Joyce M. McBeth (2018) CC BY 4.0 view source
2.2.5 Lustre
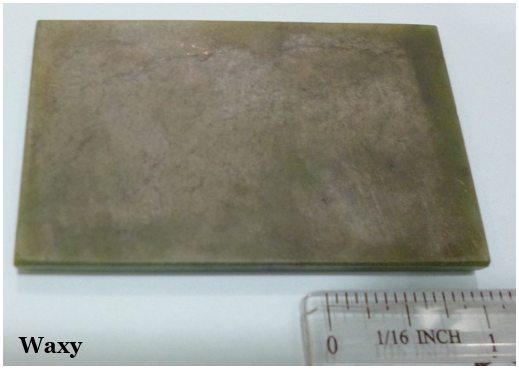
Lustre refers to the appearance of the reflection of light from a mineral’s surface. It is generally broken into two main types: metallic and non-metallic. Minerals with a metallic lustre have the colour of a metal, like silver, gold, copper, or brass (e.g. Figure 2.12). While minerals with a metallic lustre are often shiny, not all shiny minerals are metallic. Make sure you look for the colour of a metal, rather than for just a shine. Minerals with non-metallic lustre do not appear like metals. They may be vitreous or glassy (e.g. Figure 2.13A), earthy or dull (e.g. Figure 2.13B), waxy (e.g. Figure 2.13C), greasy or oily, etc.
Source: Randa Harris (2015) CC BY-SA 3.0 view source

Source: Randa Harris (2015) CC BY-SA 3.0 view source

Source: Randa Harris (2015) CC BY-SA 3.0 view source
Source: Randa Harris (2015) CC BY-SA 3.0 view source
2.2.6 Streak
Streak is an easily detectable physical property. It refers to the colour left behind on an unglazed piece of porcelain when a mineral is rubbed along its surface. A streak plate is included in your rock and mineral kit to test this property. Often a mineral will have a streak of a different colour than the mineral (e.g. Figure 2.14). Some minerals will have a white streak, which is difficult to see along the white streak plate. If you rub a mineral along the streak plate and do not see an obvious streak, wipe your finger along the streak plate; a mineral with a white streak will leave a white powder behind that will rub on your finger (e.g. Figure 2.15). Alternatively, you may use the black streak plate, which was provided in your mineral identification kit.

Source: Randa Harris (2015) CC BY-SA 3.0 view source

Source: Randa Harris (2015) CC BY-SA 3.0 view source
2.2.7 Special Physical Properties
Minerals may be magnetic, and this property is simply tested by seeing if your nail is attracted to a mineral. Magnetite is an example of a magnetic mineral. The mineral halite is simply table salt, so it will taste salty. Sphalerite will release a sulfurous smell when streaked, and talc will feel soapy when touched.
Specific gravity is the ratio of a mineral’s weight to the weight of an equal volume of water. A mineral with a specific gravity of 2 would weigh twice as much as water. Most minerals are heavier than water, and the average specific gravity for all minerals is approximately 2.7. Some minerals are quite heavy, such as pyrite with a specific gravity of 4.9-5.2, native copper, with a specific gravity of 8.8-9.0, and native gold at 19.3, which makes panning useful for gold, as the heavy mineral stays behind as you wash material out of the pan.
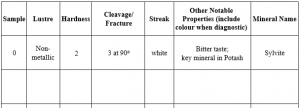
2-E1 LAB EXERCISES – MINERAL IDENTIFICATION
This lab contains 27 numbered mineral samples, labelled 1 – 27, separated into drawers. Use these instructions to test and identify them. You will test for different properties after learning about them, then work on identification at the end of the lab. As you identify properties of each sample, fill in the table provided in this lab and complete the multiple choice questions to test your knowledge. It is recommended that you obtain a mineral identification kit from the campus bookstore. It will contain:
- A copper coin
- Glass plate (wrapped in paper) – this will be used in testing hardness
- Zinc coated nail
- Unglazed porcelain plate – this will be used as a streak plate
- Black streak plate – this will be used to identify white streaks
- Hydrochloric acid bottle (empty)
- Magnifying glass (10x). To use this, hold it very close to your eye and bring the sample near the glass until it is in focus (approximately one inch from your eye).
- Magnet – this will be used to test for magnetism in minerals
Take the drawers provided by your TA and view the minerals, numbered 1-27; there are 21 different minerals provided, so expect to see some repeat minerals. We will first examine hardness from these samples and will answer more questions about them later in the lab. Look closely at each of the minerals, using the hand lens to observe them.
You need to experiment with each sample to test for its hardness, using Figure 2.5 for reference. Remember that hardness is determined by scratching the mineral. First, decide which minerals have a hardness greater than 5.5 (the hardness of glass). Lay the glass on a flat surface, then try to scratch it with each mineral by pressing down hard with the mineral. Table 2.1 is provided for you to make notations about each mineral. Note that you do not have to fill in every physical property for every mineral, just fill in the properties you are asked about as you work. Note on the table which minerals have a hardness greater than 5.5. You may also test samples by using materials to scratch them. The copper coin has a hardness of 3; any mineral that it can scratch will have a hardness less than 3. You can further refine this by using your fingernail (only natural fingernails work for this), which has an approximate hardness of 2.5, so if both the copper coin and your fingernail scratches it, you know its hardness must be <2.5. Additionally, you can use a zinc coated nail, which has a hardness of 4, and other minerals to test the hardness. For example, if you have two minerals that have a hardness of <2.5, you can see if one will scratch the other, then you know which mineral is harder.
Source: Lyndsay Hauber (2018) CC BY 4.0, after Randa Harris (2015) CC BY-SA 3.0 view source
- Sample 11: What is this sample’s hardness?
- harder than glass
- softer than glass but harder than nail
- softer than nail but harder than copper
- softer than copper but harder than a fingernail
- softer than a fingernail
- Sample 25: What is this sample’s hardness?
- harder than glass
- softer than glass but harder than nail
- softer than nail but harder than copper
- softer than copper but harder than a fingernail
- softer than a fingernail
- Sample 12: What is this sample’s hardness?
- harder than glass
- softer than glass but harder than nail
- softer than nail but harder than copper
- softer than copper but harder than a fingernail
- softer than a fingernail
- Sample 26: What is this sample’s hardness?
- harder than glass
- softer than glass but harder than nail
- softer than nail but harder than copper
- softer than copper but harder than a fingernail
- softer than a fingernail
- Sample 10: What is this sample’s hardness?
- harder than glass
- softer than glass but harder than nail
- softer than nail but harder than copper
- softer than copper but harder than a fingernail
- softer than a fingernail
- Sample 19. What is this sample’s hardness?
- harder than glass
- softer than glass but harder than nail
- softer than nail but harder than copper
- softer than copper but harder than a fingernail
- softer than a fingernail
- Sample 27. What is this sample’s hardness?
- harder than glass
- softer than glass but harder than nail
- softer than nail but harder than copper
- softer than copper but harder than a fingernail
- softer than a fingernail
- Sample 24: This sample has:
- no cleavage (it fractures)
- 1 cleavage plane
- 2 cleavage planes at 90o
- 3 cleavage planes at 90o (cubic)
- 4 cleavage planes
- Sample 24: What other unique property does this sample have?
- effervescence in acid
- it is magnetic
- it tastes salty
- it feels soapy
- it smells like sulfur
- Sample 21: This sample has:
- no cleavage (it fractures)
- 1 cleavage plane
- 2 cleavage planes at 90o
- 3 cleavage planes that are not at 90o (rhombohedral)
- 4 cleavage planes
- Sample 15: This sample has:
- no cleavage (it fractures)
- 1 cleavage plane
- 2 cleavage planes at 90o
- 3 cleavage planes at 90o
- 4 cleavage planes
- Sample 13: This sample has:
- no cleavage (it fractures)
- 1 cleavage plane
- 2 cleavage planes at 90o
- 3 cleavage planes at 90o
- 4 cleavage planes
- Sample 18: This sample has:
- no cleavage (it fractures)
- 1 cleavage plane
- 2 cleavage planes at 90o
- 3 cleavage planes at 90o
- 4 cleavage planes
- Sample 18: What is this sample’s hardness?
- harder than glass
- similar to glass
- softer than nail but harder than copper
- softer than copper but harder than a fingernail
- softer than a fingernail
- Sample 20: This sample has:
- no cleavage (it fractures)
- 1 cleavage plane
- 2 cleavage planes at 90o
- 3 cleavage planes at 90o
- 4 cleavage planes
- Sample 16: This sample has:
- no cleavage (it fractures)
- 1 cleavage plane
- 2 cleavage planes at 90o
- 3 cleavage planes at 90o
- 2 cleavage planes at 60o/120o
- Sample 16: What is this sample’s hardness?
- harder than glass
- softer than glass but harder than nail
- softer than nail but harder than copper
- softer than copper but harder than a fingernail
- softer than a fingernail
- Sample 17: This sample has:
- no cleavage (it fractures)
- 1 cleavage plane
- 2 cleavage planes at 90o
- 3 cleavage planes at 90o
- 4 cleavage planes
- Sample 22: What is this sample’s hardness?
- harder than glass
- softer than glass but harder than nail
- softer than nail but harder than copper
- softer than copper but harder than a fingernail
- softer than a fingernail
- Sample 22: This sample has:
- no cleavage (it fractures)
- 1 cleavage plane
- 2 cleavage planes at 90o
- 3 cleavage planes at 90o
- 4 cleavage planes
- Sample 4: What is the streak of this sample?
- dark grey streak
- white streak
- reddish brown streak
- pale yellow streak
- Sample 4: What is the lustre of this sample?
- non-metallic, vitreous
- non-metallic, earthy
- non-metallic, resinous
- non-metallic, waxy
- metallic
- Sample 1: What is the lustre of this sample?
- non-metallic, vitreous
- non-metallic, earthy
- non-metallic, resinous
- non-metallic, waxy
- metallic
- Sample 6: What is the streak of this sample?
- dark grey to black streak
- grey streak
- reddish brown streak
- pale yellow streak
- Sample 6: Which other item(s) is/are characteristic(s) of this sample?
- very heavy
- harder than glass
- metallic lustre
- both a and b
- both a and c
- Sample 8: What is the streak of this sample?
- dark grey to black streak
- grey streak
- reddish brown streak
- pale yellow streak
- Sample 8: What is the lustre of this sample?
- non-metallic, vitreous
- non-metallic, earthy
- non-metallic, greasy
- non-metallic, waxy
- metallic
- Sample 9: What is the lustre of this sample?
- non-metallic, vitreous
- non-metallic, earthy
- non-metallic, greasy
- non-metallic, waxy
- metallic
- Sample 9: Due to its appearance, this sample has often been confused with native gold, a mineral with a hardness of 2.5-3. How does its hardness compare with that of gold?
- Sample 9 is harder than gold.
- Sample 9 is softer than gold.
- Sample 14: What is the lustre of this sample?
- non-metallic, vitreous
- non-metallic, earthy
- non-metallic, greasy
- non-metallic, waxy
- metallic
- Sample 2: What is the streak of this sample?
- dark grey to black streak
- grey streak
- reddish brown streak
- pale yellow streak
- Sample 2: What another unique property does this sample have?
- effervescence in acid
- it is magnetic
- it tastes salty
- it feels soapy
- it smells like sulfur
- Sample 5: Examine this entire sample closely with a hand lens. What is the lustre of this sample?
- non-metallic, vitreous
- non-metallic, earthy
- non-metallic, greasy
- non-metallic, waxy
- metallic
- Sample 23: What is the streak of this sample?
- dark grey to black streak
- white streak
- reddish brown streak
- pale yellow streak
- Sample 7: What is the lustre of this sample?
- non-metallic, vitreous
- non-metallic, earthy
- non-metallic, resinous
- non-metallic, waxy
- metallic
- Sample 7: What is the streak of this sample?
- dark grey to black streak
- grey streak
- yellow-brown to brown-black streak
- pale yellow streak
- Sample 3: What is the streak of this sample?
- dark grey to black streak
- grey streak
- reddish brown streak
- pale yellow streak
- Sample 3: What is the lustre of this sample?
- non-metallic, vitreous
- non-metallic, earthy
- non-metallic, greasy
- non-metallic, waxy
- metallic
- Sample 1: What is this sample?
- chalcopyrite
- limonite
- galena
- sphalerite
- magnetite
- hematite
- Sample 2: What is this sample?
- chalcopyrite
- limonite
- galena
- sphalerite
- magnetite
- hematite
- Sample 2: What other unique property does this sample have?
- effervescence in acid
- it is magnetic
- it tastes salty
- it feels soapy
- it smells like sulfur
- Sample 3: What is this sample?
- chalcopyrite
- limonite
- galena
- sphalerite
- magnetite
- hematite
- Sample 4: What is this sample?
- chalcopyrite
- limonite
- galena
- sphalerite
- magnetite
- hematite
- Sample 4: What other unique property does this sample have?
- effervescence in acid
- it is magnetic
- it tastes salty
- it feels soapy
- it smells like sulfur
- Sample 5: What is this sample?
- chalcopyrite
- limonite
- galena
- sphalerite
- magnetite
- hematite
- Sample 6: What is this sample?
- chalcopyrite
- limonite
- galena
- sphalerite
- magnetite
- hematite
- Sample 7: What is this sample?
- chalcopyrite
- limonite
- galena
- sphalerite
- magnetite
- hematite
- Sample 7: What other unique property does this sample have?
- effervescence in acid
- it is magnetic
- it tastes salty
- it feels soapy
- it smells like sulfur
- Sample 8: What is this sample?
- chalcopyrite
- limonite
- galena
- pyrite
- magnetite
- hematite
- Sample 9: What is this sample?
- chalcopyrite
- limonite
- galena
- pyrite
- magnetite
- hematite
- Sample 10: What is this sample?
- quartz
- muscovite
- potassium feldspar
- calcite
- biotite
- plagioclase feldspar
- Sample 11: What is this sample?
- quartz
- muscovite
- potassium feldspar
- calcite
- biotite
- plagioclase feldspar
- Sample 12: What is this sample?
- quartz
- muscovite
- potassium feldspar
- calcite
- biotite
- plagioclase feldspar
- Sample 13: What is this sample?
- quartz
- muscovite
- potassium feldspar
- calcite
- biotite
- plagioclase feldspar
- Sample 14: What is this sample?
- quartz
- muscovite
- potassium feldspar
- calcite
- biotite
- plagioclase feldspar
- Sample 15: What is this sample?
- quartz
- muscovite
- potassium feldspar
- calcite
- biotite
- plagioclase feldspar
- Sample 16: What is this sample?
- quartz
- amphibole
- potassium feldspar
- olivine
- pyroxene
- plagioclase feldspar
- Sample 17: What is this sample?
- quartz
- amphibole
- potassium feldspar
- olivine
- pyroxene
- plagioclase feldspar
- Sample 18: What is this sample?
- quartz
- amphibole
- potassium feldspar
- olivine
- pyroxene
- plagioclase feldspar
- Sample 19: What is this sample?
- quartz
- amphibole
- potassium feldspar
- olivine
- pyroxene
- plagioclase feldspar
- Sample 20: What is this sample?
- quartz
- amphibole
- potassium feldspar
- olivine
- pyroxene
- plagioclase feldspar
- Sample 21: What is this sample?
- quartz
- gypsum
- cryptocrystalline quartz
- calcite
- halite
- plagioclase feldspar
- Sample 22: What is this sample?
- quartz
- gypsum
- cryptocrystalline quartz
- calcite
- halite
- plagioclase feldspar
- Sample 23: What is this sample?
- quartz
- gypsum
- cryptocrystalline quartz
- calcite
- halite
- plagioclase feldspar
- Sample 24: What is this sample?
- talc
- gypsum
- cryptocrystalline quartz
- garnet
- halite
- plagioclase feldspar
- Sample 24: What other unique property does this sample have?
- it effervescence in acid
- it is magnetic
- it tastes salty
- it feels soapy
- it smells like sulfur
- Sample 25: What is this sample?
- talc
- gypsum
- cryptocrystalline quartz
- garnet
- halite
- plagioclase feldspar
- Sample 26: What is this sample?
- talc
- gypsum
- cryptocrystalline quartz
- garnet
- halite
- plagioclase feldspar
- Sample 26: What other unique property does this sample have?
- it effervescence in acid
- it is magnetic
- it tastes salty
- it feels soapy
- it smells like sulfur
- Sample 27: What is this sample?
- talc
- gypsum
- cryptocrystalline quartz
- garnet
- halite
- plagioclase feldspar

