7. Muscle
Chapter 7. Muscle, and the Neuromuscular Junction
7.1 Introduction
7.1.1 Three Kinds of Muscle
7.2 Striated Muscle
7.2.1 The Contractile Machinery
7.2.2 Control by the Somatic Nervous System
7.2.3 Coupling of Contraction to Excitation
7.2.4 Tension and Load
7.2.5 The Energy Supply
7.3 Smooth Muscle
7.3.1 Coupling of Contraction to Excitation
7.3.2 Neuromodulation of Smooth Muscle Activity
7.4 Summary
7.5 Suggested Readings and Viewings
7.6 Glossary
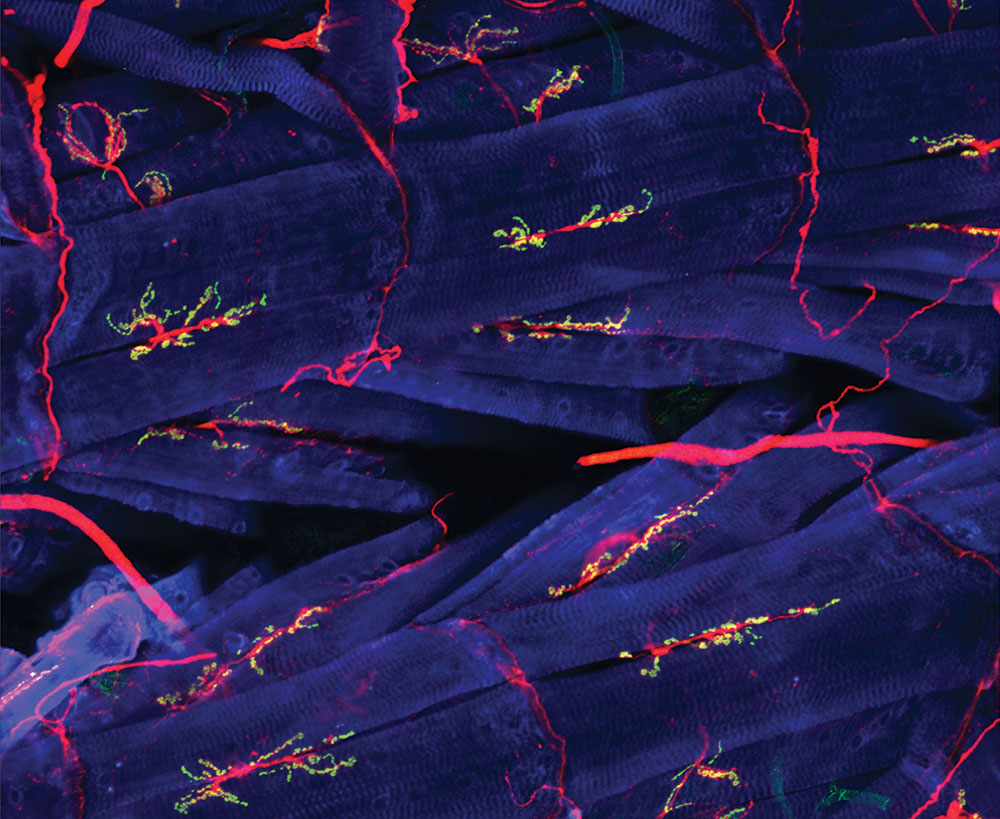 Figure 7.1 Neuromuscular junctions in the fly. Blue: muscle cells. Red: axons. Green: synapses. Source: NICHD / National Institutes of Health / Y.J. Kim and M. Serpe, https://www.flickr.com/photos/nichd/17134418700 CC BY 2.0, 2015.
Figure 7.1 Neuromuscular junctions in the fly. Blue: muscle cells. Red: axons. Green: synapses. Source: NICHD / National Institutes of Health / Y.J. Kim and M. Serpe, https://www.flickr.com/photos/nichd/17134418700 CC BY 2.0, 2015.
7.1 Introduction
Usain St Leo Bolt of Jamaica and Kenenisa Bekele of Ethiopia are considered to be among the greatest runners of all time. But you never see them in the same race, even though they’re roughly the same age. Why?
Usain Bolt achieved world records for the 100 metre and 200 metre races, but has never competed in a major official race longer than 400 metres. And Kenenisa Bekele achieved world records for the 2000 metre, 5000 metre, and 10,000 metre races, but has never competed in a major official race shorter than 800 metres. We all know that exercise improves muscle performance, so why are the best sprinters not good longer distance runners, and vice versa? What makes the leg muscle of a champion sprinter different from the leg muscle of a long distance runner?
Have you ever had a traditional North American Thanksgiving? Have you attended one of the popular community fall dinners in rural Saskatchewan? If so, you’ll know that muscle looks different based on the kind of exercise it has had, even if the amount of exercise is the same: The flight muscles of turkey breast are “white meat”, adapted for short, anaerobic bursts of flight. And the leg muscles are mostly “dark meat”, adapted for running around.
List the differences between animals and plants. Probably close to the top of your list: That animals actively move. Even if an animal sits still, organs inside the body are actively moving, under muscular power. While this isn’t true of all animals, muscle is an important tissue in most. Indeed, more than a third of our body mass is muscle, making muscle the most abundant tissue in the human body. If we want to understand how the body works, we need to know how muscle communicates with the rest of the body and adapts to the body’s needs.
How are muscular actions co-ordinated? In this chapter, we’ll look at how the central nervous system (CNS) controls movement in the animal body. In humans and other extremely cephalized animals, motor functions can be divided into two general categories: Muscle action that you’re consciously aware of, and muscle action you’re not consciously aware of, not directly. The CNS uses two branches of the peripheral nervous system (PNS) to control these motions. Through the somatic nervous system (SNS), the CNS controls conscious movements, while through the autonomic nervous system (ANS), the CNS controls involuntary functions, such as activity of cardiac (heart) muscles, and muscles in the walls of blood vessels and internal organs.
In all but the very simplest animals, there are cells and tissues that are specialized for exerting force by contracting. These are called muscle. Muscle is a soft and electrically excitable tissue specialized for contraction. It is found in many different organs with a variety of shapes and functions.
7.1.1 Three Kinds of Muscle
Based on how the muscle looks under a microscope, muscle tissue can be classified into two different types: striated and smooth. Striated muscle gets its name from its striped appearance under a microscope. Striated muscle has stripes because the contractile units within the muscle cells are lined up with each other. Based on function, striated muscle can be further classified into striated voluntary muscle and striated non-voluntary muscle (Figures 7.2 and 7.3).
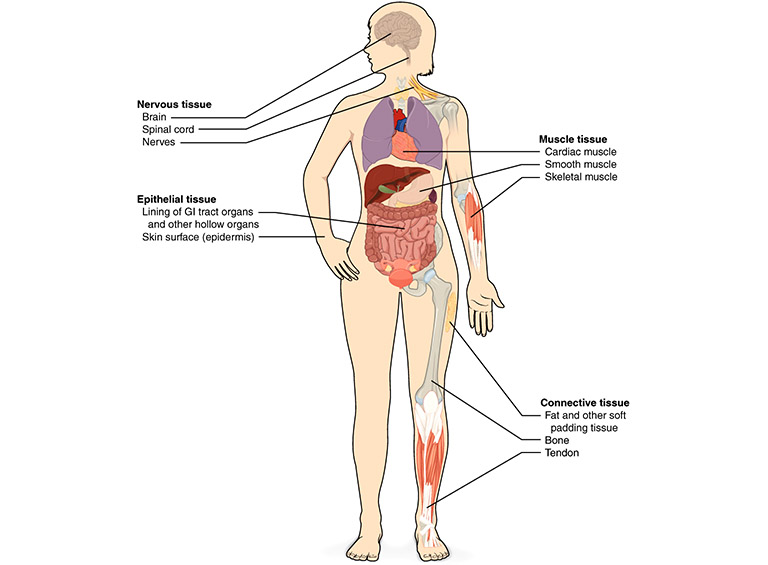 Figure 7.2 Tissues in the human body. Source: adapted from Anatomy & Physiology, Rice University, download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@6.27 CC BY 3.0, 2013.
Figure 7.2 Tissues in the human body. Source: adapted from Anatomy & Physiology, Rice University, download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@6.27 CC BY 3.0, 2013.
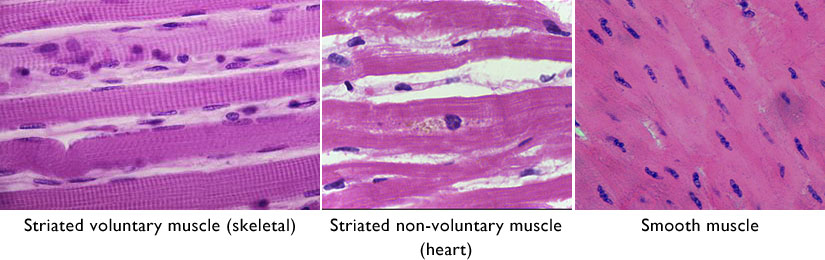 Figure 7.3 Three kinds of muscle tissue. Sources: adapted from Rollroboter / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Muskel_(_1).jpg CC BY-SA 3.0, 2013; Department of Pathology, Calicut Medical College, Kozhikode, https://commons.wikimedia.org/wiki/File:Cardiac_myocyte_showing_lipofuscin_pigment.jpg CC BY-SA 4.0, 2014; Andrea Scauri / (biophotos), https://www.flickr.com/photos/andrea_scauri/2969518547 CC BY 2.0, 2008.
Figure 7.3 Three kinds of muscle tissue. Sources: adapted from Rollroboter / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Muskel_(_1).jpg CC BY-SA 3.0, 2013; Department of Pathology, Calicut Medical College, Kozhikode, https://commons.wikimedia.org/wiki/File:Cardiac_myocyte_showing_lipofuscin_pigment.jpg CC BY-SA 4.0, 2014; Andrea Scauri / (biophotos), https://www.flickr.com/photos/andrea_scauri/2969518547 CC BY 2.0, 2008.
Striated voluntary muscle includes the majority of the muscles of the body involved in locomotion, stretching, chewing, breathing, and maintaining balance. Striated voluntary muscle is mostly found attached at both ends to bones through flexible but tough bands of fibrous connective tissue made of collagen. These bands of connective tissue are called tendons. Some skeletal muscles can also be attached to other muscles or to connective tissue under the skin. In humans, each striated voluntary muscle is under both conscious control and unconscious control.
Striated non-voluntary muscle is found in the heart wall, forming the myocardium, a tissue consisting of cells termed cardiomyocytes or myocardiocytes. Cardiomyocytes are mostly mononucleated (have only one nucleus per cell), although some multinucleated (multiple nuclei per cell) cardiomyocytes are also found. Contraction of the heart muscle is entirely under involuntary control. A cluster of cardiac pacemaker cells organized into the sinoatrial node[1] co-ordinates heart contractions. The sinoatrial node is itself regulated by cholinergic and adrenergic axon terminals of the autonomic nervous system (ANS) to adjust the frequency and strength of heart contractions.
Smooth muscle is not striated and is not under conscious control, and is found in organs that require sustained contraction, mostly those involved in homeostatic control of body functions. They contract blood vessels and the gastrointestinal, respiratory, reproductive and urinary tracts. Smooth muscle also controls the size of the pupil of the eye, and the shape of the eye’s lens for focusing. Tiny smooth muscles in the skin control erection hairs and feathers.
As the signalling for the skeletal muscle contractions is originated by inputs from motor neurons, muscle contraction is said to be neurogenic; while involuntary contractions that are initiated by the viscera or heart muscle itself are said to be myogenic. This chapter describes the regulation of muscle, emphasizing communication, from CNS signalling to the transformation of chemical energy into mechanical energy in muscle contraction.
7.2 Striated Muscle
The structural unit of muscle tissue is the myocyte – known as a muscle fibre in striated muscle – a long and cylindrical cell surrounded by an electrically sensitive plasma membrane, termed the sarcolemma. The sarcolemma propagates action potentials very rapidly, much like the neuron’s plasma membrane. The cytoplasm of myocytes is termed sarcoplasm.
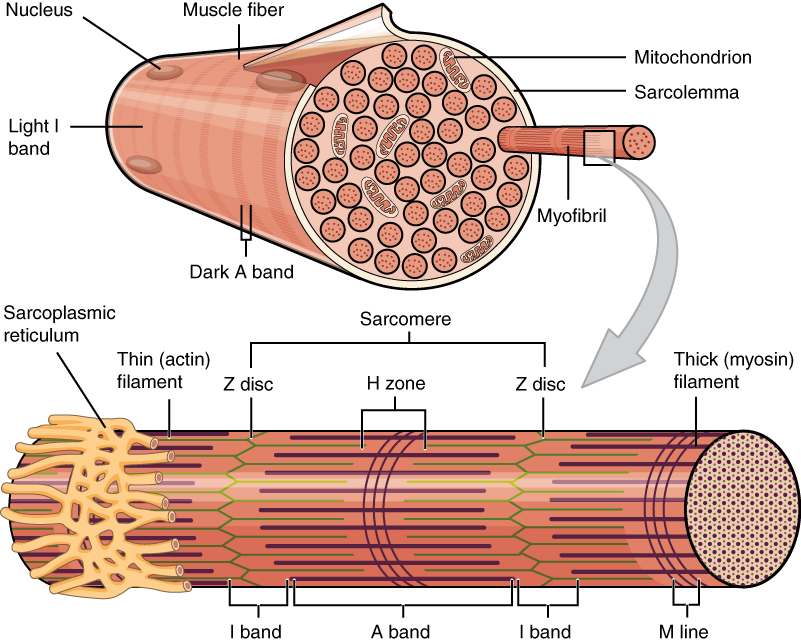 Figure 7.1 A skeletal muscle cell, or “fibre”. Source: Anatomy & Physiology, Rice University, download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.25 CC BY 4.0, 2016.
Figure 7.1 A skeletal muscle cell, or “fibre”. Source: Anatomy & Physiology, Rice University, download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.25 CC BY 4.0, 2016.
The precursors of the myocytes are mesodermal cells termed myoblasts, which during embryonic development fuse to create a multinucleated myocyte of up to 100 nuclei per cell. Mature myocytes also contain a large number of mitochondria scattered throughout, and a differentiated smooth endoplasmic reticulum termed the sarcoplasmic reticulum (SR) that stores ions and regulates their concentration in the sarcoplasm. An extension of the sarcolemma penetrates into the sarcoplasm in the form of transverse tubules (T-tubules), leaving the extracellular fluid in close contact with the SR.
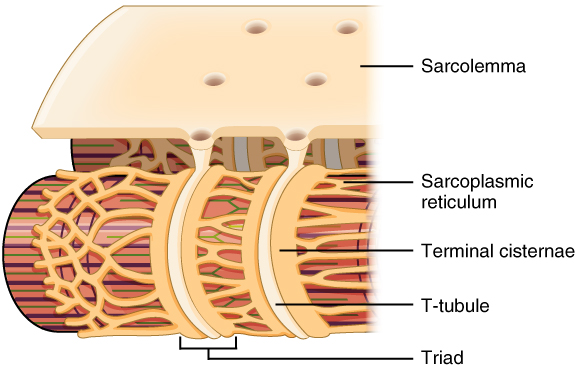 Figure 7.2 T-tubules are extensions of the plasma membrane (sarcolemma) of the muscle cell (fibre, or myocyte) into the cytoplasm (sarcoplasm) and come close to the endoplasmic reticulum (sarcoplasmic reticulum). Source: Anatomy & Physiology, Rice University, download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.25 CC BY 4.0, 2016.
Figure 7.2 T-tubules are extensions of the plasma membrane (sarcolemma) of the muscle cell (fibre, or myocyte) into the cytoplasm (sarcoplasm) and come close to the endoplasmic reticulum (sarcoplasmic reticulum). Source: Anatomy & Physiology, Rice University, download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.25 CC BY 4.0, 2016.
Striated muscle is highly vascularized, and therefore provided with an abundant supply of blood carrying oxygen. In addition, the myocytes of most skeletal muscles contain the red pigment myoglobin[2] which stores oxygen, and abundant granules of glycogen that serve as an easily available energy reserve.
The functional unit in the contracting sarcoplasm is a set of 4 to 20 rod-like filaments known as a myofibril. A myofibril is composed of a group of protein filaments that form the contractile machinery of the muscle. Myofibrils contract in response to action potentials in the sarcolemma, (described below in the Muscle Contraction section).
In striated muscle, myofibrils are organized along their length into smaller functional units termed sarcomeres (Greek, sarco: flesh; meros: segment). The repetition of sarcomeres within the muscle fibre gives the skeletal and cardiac muscles their characteristic striated appearance seen in under the microscope (Figure 7.3).
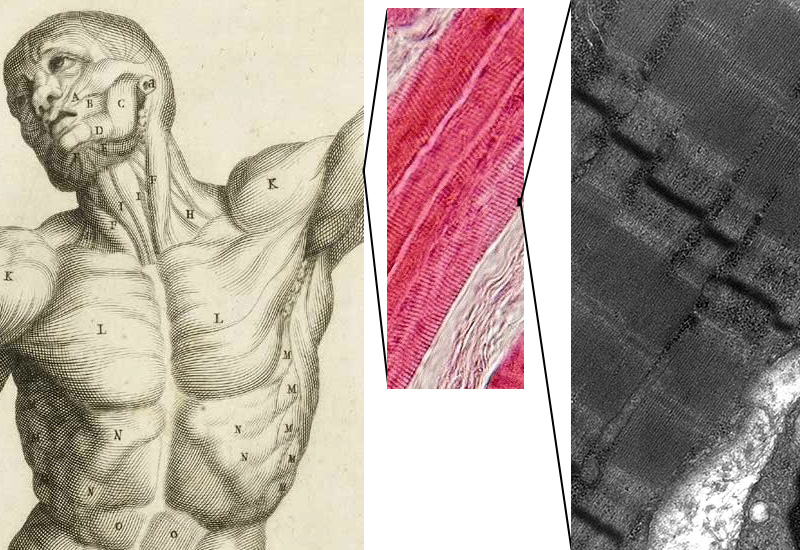 Figure 7.3 In striated muscle, the microscopic stripes are due to repeating units of machinery that contracts. Left, skeletal muscle, which is most of the striated voluntary muscle in the human body; Centre, thin section of skeletal muscle, viewed in a light microscope, proteins stained with a pink dye; Right, thin section of skeletal muscle, viewed in an electron microscope. Sources: Left, Bernardino Genga, Anatomia per Uso et Intelligenza del Disegno (Rome: Domenico de’ Rossi, 1691) CC0 Public Domain; Centre, Department of Histology, Jagiellonian University Medical College / Wikimedia Commons https://commons.wikimedia.org/wiki/File:Skeletal_muscle_-_longitudinal_section.jpg CC-BY-SA 3.0, 2006; Right, Louisa Howard, Dartmouth Electron Microscope Facility, Dartmouth College
Figure 7.3 In striated muscle, the microscopic stripes are due to repeating units of machinery that contracts. Left, skeletal muscle, which is most of the striated voluntary muscle in the human body; Centre, thin section of skeletal muscle, viewed in a light microscope, proteins stained with a pink dye; Right, thin section of skeletal muscle, viewed in an electron microscope. Sources: Left, Bernardino Genga, Anatomia per Uso et Intelligenza del Disegno (Rome: Domenico de’ Rossi, 1691) CC0 Public Domain; Centre, Department of Histology, Jagiellonian University Medical College / Wikimedia Commons https://commons.wikimedia.org/wiki/File:Skeletal_muscle_-_longitudinal_section.jpg CC-BY-SA 3.0, 2006; Right, Louisa Howard, Dartmouth Electron Microscope Facility, Dartmouth College
http://remf.dartmouth.edu/images/humanMuscleTEM/source/3.html CC0 Public Domain.
7.2.1 The Contractile Machinery
The Sarcomere The cross-striations of sarcomeres appear as a series of bands that are identified by letters, named for their optical properties under a polarizing microscope (Figure ). In skeletal and cardiac muscle, the striped appearance is due to the alternating A bands and I bands. The A band stays the same length when the sarcomere contracts during muscle contraction, while the I band contracts. When stained for proteins, the A band is much darker than the I band. The reason why the A band is darker than the I band will become clear when you look at the detailed structure of the contractile machinery (described below). The I and A bands both contain additional bands seen under the microscope. The dark A band has a lighter H band in the centre, and an M line crosses in the middle of the H band. The M line plus the narrow light areas on either side of it are called the pseudo-H zone. The light I band is divided by a narrow dark Z line. Thus, the area between two adjacent Z lines defines a sarcomere.
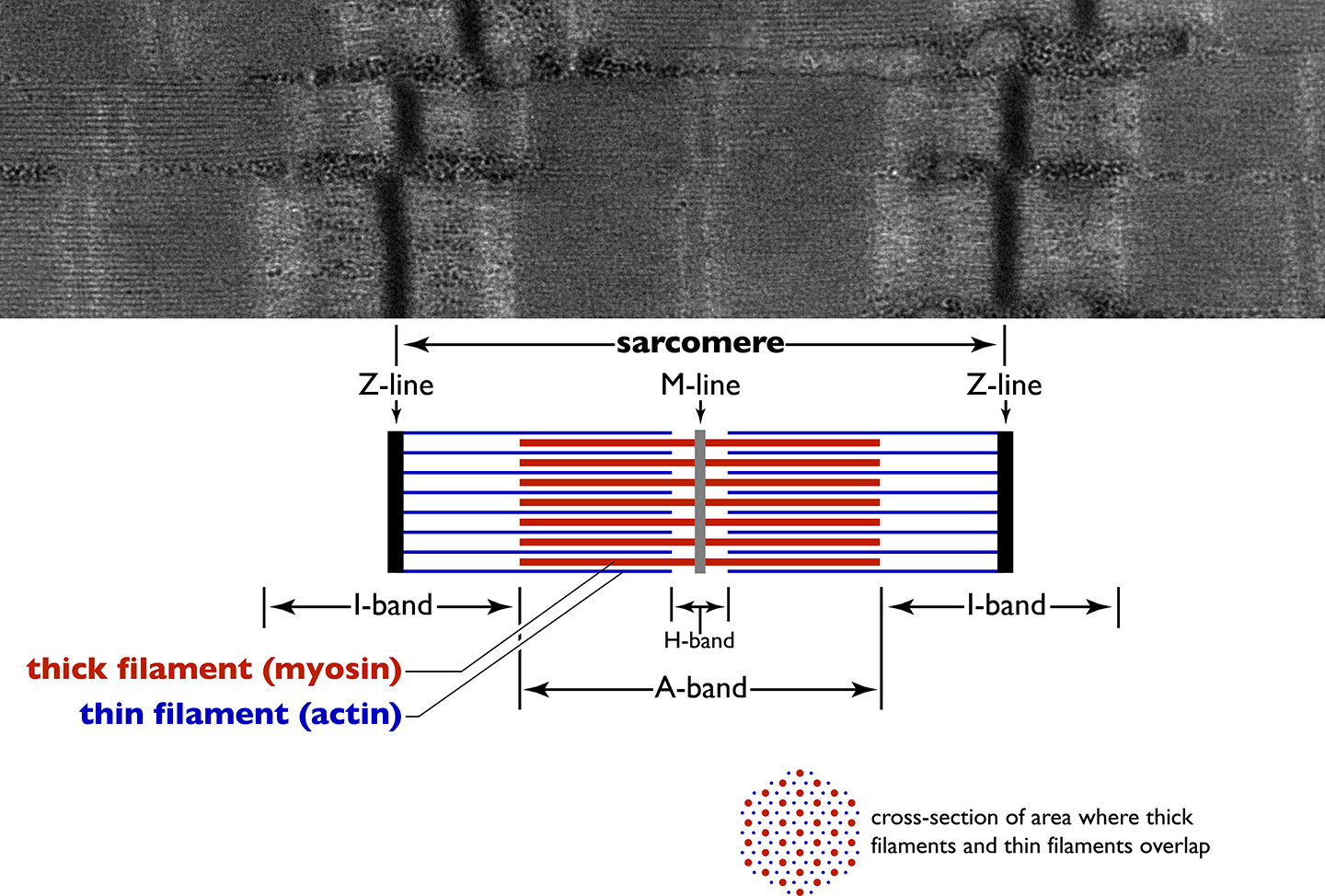 Figure 7.4 The bands seen in striated muscle correspond to the parts of the sarcomere, the working unit that creates the force of muscle contraction. Source: electron micrograph from Louisa Howard, Dartmouth Electron Microscope Facility, Dartmouth College http://remf.dartmouth.edu/images/humanMuscleTEM/source/3.html CC0 Public Domain, 2004.
Figure 7.4 The bands seen in striated muscle correspond to the parts of the sarcomere, the working unit that creates the force of muscle contraction. Source: electron micrograph from Louisa Howard, Dartmouth Electron Microscope Facility, Dartmouth College http://remf.dartmouth.edu/images/humanMuscleTEM/source/3.html CC0 Public Domain, 2004.
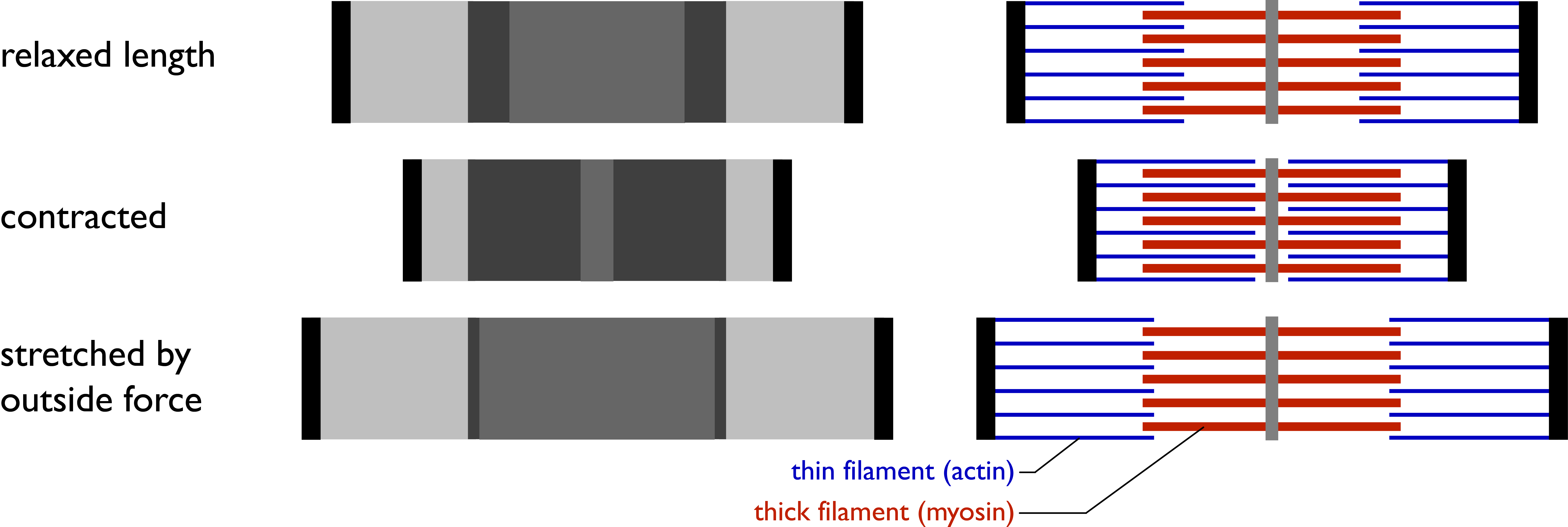 Figure 7.5 The sliding filament model for sarcomere contraction. CC0 Public Domain, 2017.
Figure 7.5 The sliding filament model for sarcomere contraction. CC0 Public Domain, 2017.
Structural Components of the Myofibrils Myofibrils contain two kinds of filaments, termed myofilaments, the thick filaments and the thin filaments.
- A thick filament is composed of parallel bundles of approximately 200 to 300 molecules of the motor protein myosin. The thick filament shows multiple levels of assembly of protein molecules into larger structures. The structure is best understood by looking at a diagram (Figure X.y). The main component is myosin heavy chain, which accounts for roughly half the protein mass of muscle tissue. Each heavy chain molecule consists of a globular “head” connected to a long α-helix “tail.” The heavy chain tail wraps around the tail of a second, identical heavy chain molecule, forming a protein dimer (Greek, dís: “twice”; méros: “part”). The tails of these dimers assemble themselves into thick filaments consisting of a hundred or more myosin heavy chain dimers. The myosin head has one binding site for ATP, and another binding site for the thin filament protein actin. The myosin head is an active ATPase, which by converting ATP into ADP and PO4 (Pi), powers the contraction system. Myosin also has light chains, small proteins that stiffen the connection between head and tail to amplify the motion of the head and transmit force to the tail.
- A thin filament is composed of two intertwining chains of the globular protein actin. Additional proteins bound to actin include tropomyosin and troponin. Tropomyosin is dimer of two α-helixes wrapped around each other to form a very elongated molecule that binds along the length of each actin chain. Troponin is a calcium-binding complex of three proteins. In the absence of calcium ions (Ca2+), troponin blocks the myosin-binding site of actin. Troponin is exclusively in cardiac and skeletal muscle, being absent in smooth muscle.
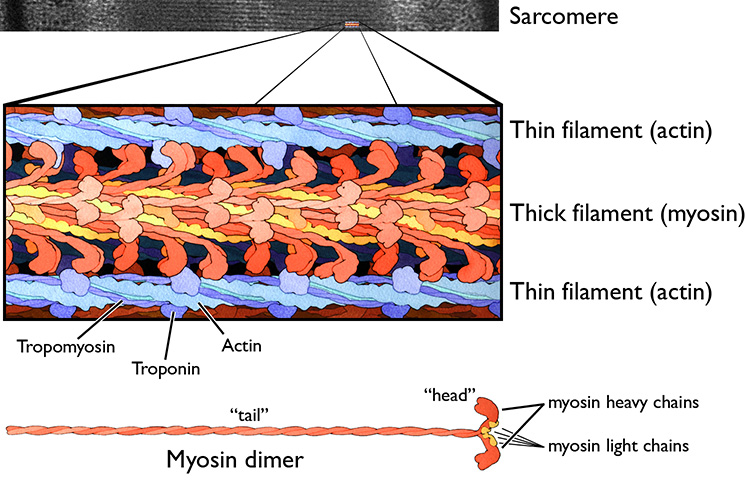 Figure 7.6 Thin filaments (polymers of the protein actin), and thick filaments (polymers of the protein myosin) are the two most important protein components of sarcomeres. Source: adapted from an electron micrograph by Louisa Howard, Dartmouth Electron Microscope Facility, Dartmouth College http://remf.dartmouth.edu/images/humanMuscleTEM/source/3.html CC 0 Public Domain, and a painting by David S. Goodsell / RCSB PDB, http://pdb101.rcsb.org/motm/18 CC BY 4.0, 2001.
Figure 7.6 Thin filaments (polymers of the protein actin), and thick filaments (polymers of the protein myosin) are the two most important protein components of sarcomeres. Source: adapted from an electron micrograph by Louisa Howard, Dartmouth Electron Microscope Facility, Dartmouth College http://remf.dartmouth.edu/images/humanMuscleTEM/source/3.html CC 0 Public Domain, and a painting by David S. Goodsell / RCSB PDB, http://pdb101.rcsb.org/motm/18 CC BY 4.0, 2001.
Other protein components of the myofibrils include titin and nebulin. Titin is a large peptide that maintains uniform tension across the sarcomere, and keeps the thick filament in a central position. Nebulin is an actin-associated protein that acts as a molecular ruler regulating the length of the actin filaments.
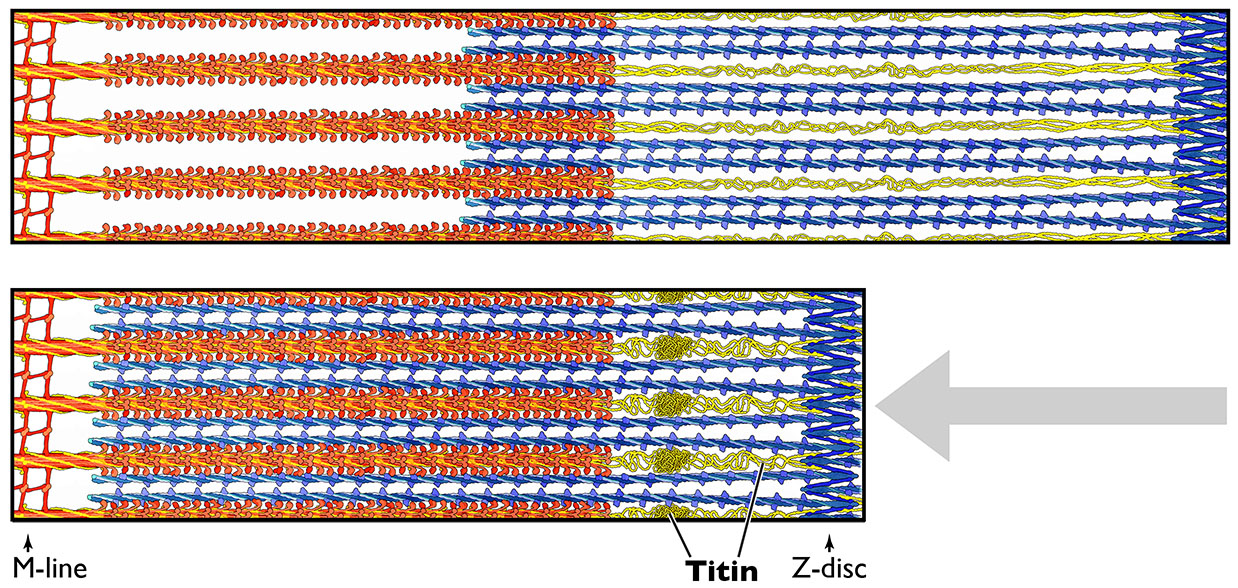 Figure 7.7 The giant protein titin acts like a spring or rubber band to hold the sarcomere structure together after stretching. Source: painting by David S. Goodsell / RCSB PDB, http://pdb101.rcsb.org/motm/185 CC BY 4.0, 2015.
Figure 7.7 The giant protein titin acts like a spring or rubber band to hold the sarcomere structure together after stretching. Source: painting by David S. Goodsell / RCSB PDB, http://pdb101.rcsb.org/motm/185 CC BY 4.0, 2015.
7.2.2 Control by the Somatic Nervous System
The SNS controls muscle contraction by generating action potentials in multipolar motor neurons, termed α-motor neurons, located in the brainstem and spinal cord. These neurons innervate multiple muscle fibres that contract in synchrony in each muscle. Axons from α-motor neurons split into a number of branches that reach the surface of individual muscle fibres in the form of bulb-shaped endings, which make contact with a specialized structure of the sarcolemma, termed the motor end-plate. The axon terminal and the end plate form a functional structure termed the neuromuscular junction (NMJ), that functions like a synapse where motor neuron axons release the neurotransmitter acetylcholine (ACh). Unlike most neurons, somatic efferent motor neurons that innervate skeletal muscle are always excitatory.
Efferent signals from the α-motor neurons rapidly propagate by saltatory[3] conduction to reach the NMJ, causing influx of Ca2+ ions through the voltage-gated calcium channels in the axon terminal. Ca2+ influx causes synaptic vesicles containing ACh to fuse with the plasma membrane, releasing the neurotransmitter by exocytosis into the synaptic cleft of the motor end plate. ACh binds and activates ionotropic nicotinic ACh receptors (nAChR) on the sarcolemma side (end plate) of the NMJ.
The nAChR is a sodium/potassium ligand-binding channel that responds to ACh with a large increase in the entrance of Na+ compared to a smaller movement of K+ outwards of the muscle fibre. As Na+ enters the cell, the sarcolemma depolarizes, reducing the potential to threshold, initiating an action potential that propagate throughout the sarcolemma. The action potential reverses the sarcolemma’s polarity from -90mV to as high as +75mV. Depolarization of the sarcolemma starts a cascade of reactions that triggers muscle contraction. When K+ exits the cell, the sarcolemma hyperpolarizes getting back to resting membrane potential. Meanwhile, ACh is degraded by acetylcholinesterase, a very active enzyme present on the axonal side of the motor-end plate membrane, terminating muscle fibre contraction.
Curare is a common name for various poisons originating from plants of South America. It is a non-depolarizing muscle relaxant that blocks the nicotinic acetylcholine receptor (nAChR). The main toxin of curare, d-tubocurarine, occupies the same site on the receptor as ACh with an equal or greater affinity, but d-tubocurarine elicits no response, making it a competitive antagonist.
7.2.3 Coupling of Contraction to Excitation
Excitation–contraction coupling is the process by which an electrical signal is converted into the mechanical action of muscle contraction. This process starts at the sarcolemma by activation of two Ca2+ channels:
- The voltage-gated L-type Ca2+ channels, also called dihydropyridine receptors (DHPRs), located in the sarcolemma; and
- The SR-Ca2+ release channels, also called ryanodine receptors (RyRs), located across the SR membrane.
In a muscle fibre, calcium ions are stored inside of the SR cisternae reversibly bound to the protein calsequestrin. Depolarization of the sarcolemma spreads across the cell surface where it activates the DHPRs, and to the SR membrane through the T-tubules where it activates the RyR. Ca2+ floods out of the cisternae into the sarcoplasm, raising its concentration from 0.1 µmol/L to 10 µmol/L. This increased concentration of cytoplasmic Ca2+ in the sarcoplasm saturates the calcium binding sites on troponin associated with actin filaments, allowing myosin to bind to actin, which in turn allows the force-producing cycle to proceed.
The mechanism of myofilament contraction is a dynamic cycle of repetitive events by which the thin filaments slide over the thick filaments, generating the force that causes muscle contraction. This process is known as the sliding filament mechanism. This process can be divided into four steps:
- Myosin, loaded with ADP and Pi from the previous round of the cycle, binds firmly to actin. This forms the complex actin-myosin-ADP-Pi, where the myosin head forms a crossbridge between the thick filament (myosin) and thin filament (actin).
- The binding of myosin to actin causes the myosin head to change in shape. This change in shape makes the cross-bridge rotate and tilt ahead, moving the actin filament forward. This is called the power stroke step. The change in the myosin head’s shape also opens up the nucleotide-binding pocket of myosin, releasing the bound Pi and ADP.
- Now that the nucleotide-binding site is empty, a new molecule of ATP binds to myosin, releasing myosin from actin, thus breaking the cross bridge.
- The myosin head ATPase hydrolyzes the bound ATP, moving myosin head back into a “cocked” position. ATP hydrolysis provides the energy to drive the cycle forwards, and the change in the myosin head’s shape stores mechanical energy to be released in the power stroke later on, like the spring of a mousetrap. With the ATP hydrolyzed, the cocked myosin head now contains ADP + Pi.
Thus, ATP plays two crucial functions in the cross-bridge cycle: it provides energy for the movement of the cross-bridge, and it breaks the bond between actin and myosin permitting the repetition of the contraction cycle as the thick and thin myofilaments slide past each other.
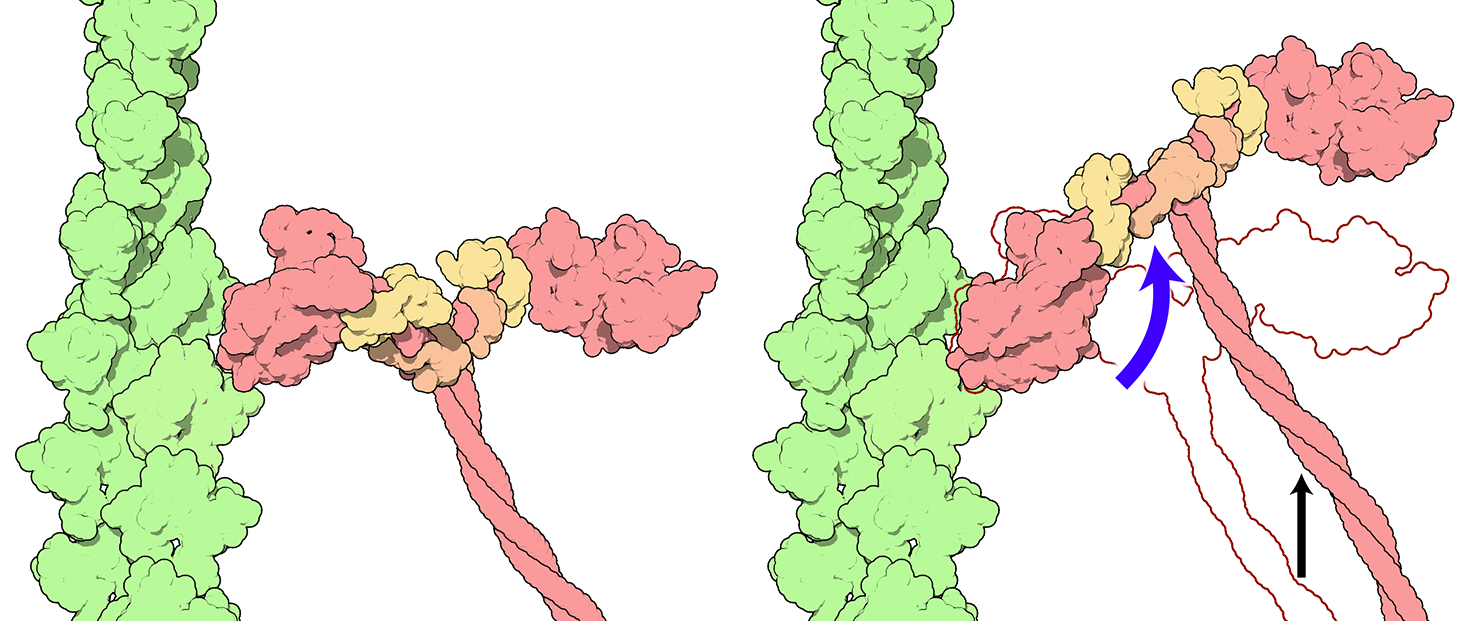 Figure 7.8 The power stroke, where the myosin head releases stored mechanical energy by changing shape, causes the thick filament to slide relative to the thin filament. Blue arrow: the power stroke. Black arrow: motion of myosin relative to actin. Source: adapted from David S. Goodsell / RCSB PDB, http://pdb101.rcsb.org/motm/18 CC BY 4.0, 2001.
Figure 7.8 The power stroke, where the myosin head releases stored mechanical energy by changing shape, causes the thick filament to slide relative to the thin filament. Blue arrow: the power stroke. Black arrow: motion of myosin relative to actin. Source: adapted from David S. Goodsell / RCSB PDB, http://pdb101.rcsb.org/motm/18 CC BY 4.0, 2001.
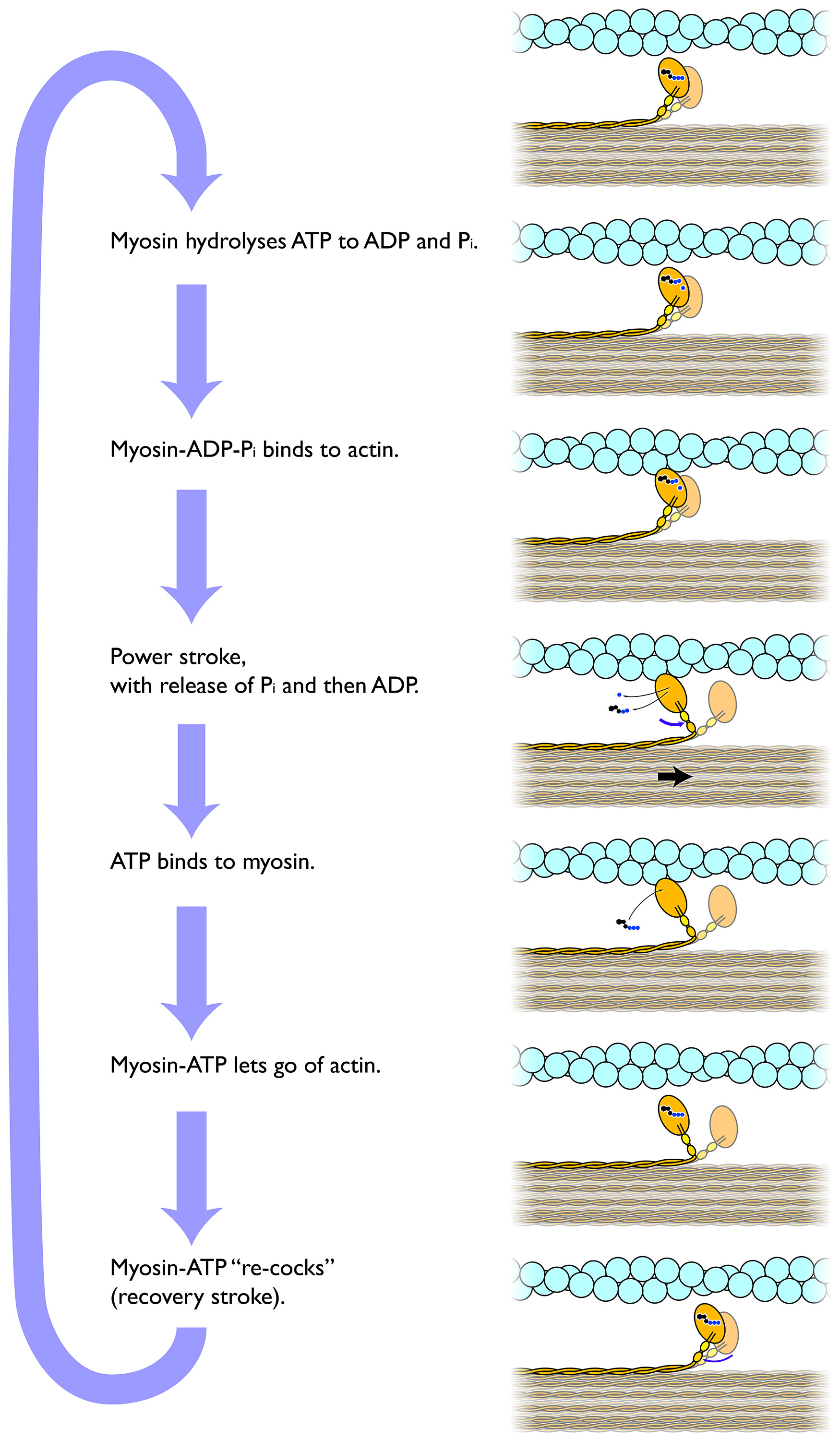 Figure 7.9 The crossbridge cycle driving sarcomere contraction. CC0 Public Domain, 2017.
Figure 7.9 The crossbridge cycle driving sarcomere contraction. CC0 Public Domain, 2017.
The end of muscle contraction is called muscle relaxation, when the muscle fibres return to a low-tension state. The duration of contraction depends on the rate at which the SR pumps Ca2+ back into the cisternae. As Ca2+ returns to resting levels, the contraction force declines and muscle relaxes. Ca2+ is returned to the sarcoplasmic reticulum by a calcium-ATPase. When the muscle is relaxed, tropomyosin blocks the attachment sites for the myosin cross bridges (heads), thus preventing contraction.
7.2.4 Tension and Load
Sliding of myofilaments generates a force that would shorten a muscle in the absence of an opposing force. Muscle forces can be distinguished between tension and load. Tension is the force that a muscle exerts on an object, while load is the force exerted by an object on the muscle.
- When the muscle shortens, the contraction is termed concentric contraction (eg. muscle lifting a weight). This happens when the tension is greater than the load.
- When the muscle lengthens, the contraction is termed isotonic eccentric contraction (for example, gently setting an object down). This happens when the tension is less than the load.
- When muscle tension stays constant during a change in length, muscle contraction is called isotonic. Both concentric and eccentric contractions can be isotonic.
- When muscle tension causes that length to remain the same due to an opposing force, muscle contraction is called isometric (Do not confuse with isotonic contractions!) In isometric contraction, the force of the muscle equals the load on the muscle (eg. muscle holding a weight in the same position).
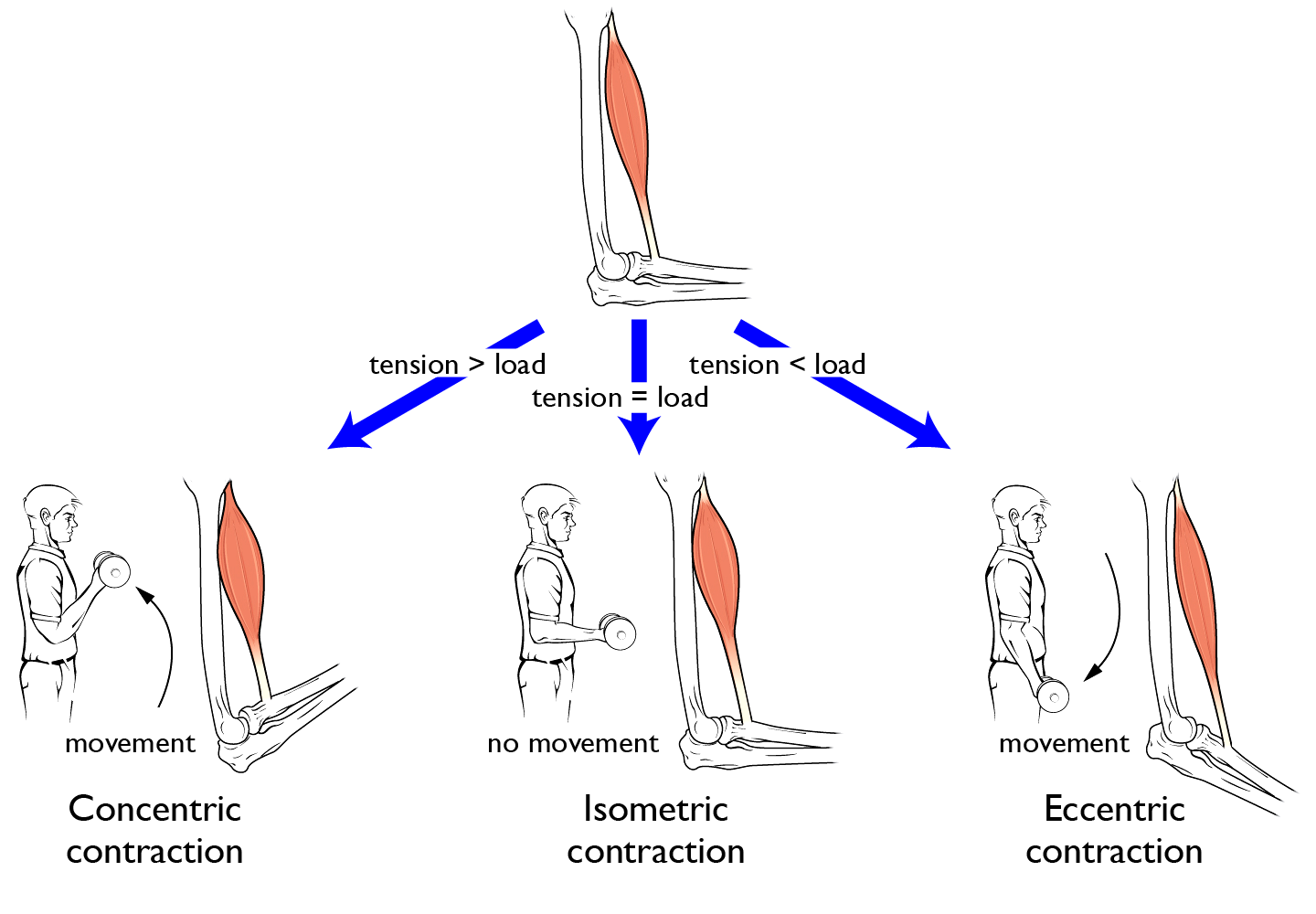 Figure 7.10 Muscle can actively exert force regardless of whether the muscle gets shorter, stays the same length, or gets longer due to the opposing force. Source: adapted from Anatomy & Physiology, Rice University, download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.25 CC BY 4.0, 2016.
Figure 7.10 Muscle can actively exert force regardless of whether the muscle gets shorter, stays the same length, or gets longer due to the opposing force. Source: adapted from Anatomy & Physiology, Rice University, download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.25 CC BY 4.0, 2016.
Depending on the frequency of the action potentials, muscular contraction can be described as follows:
- Twitch is the muscular response to a single action potential;
- Summation is the muscular response to a second stimulus before a relaxation is complete. Muscle summation adds force to the previous stimulus, developing greater tension;
- Tetanus is the muscular response to a high rate of action potentials, high enough to prevent muscle relaxation between each stimulus. Thus, during tetanus, muscle remains in a contracted state.
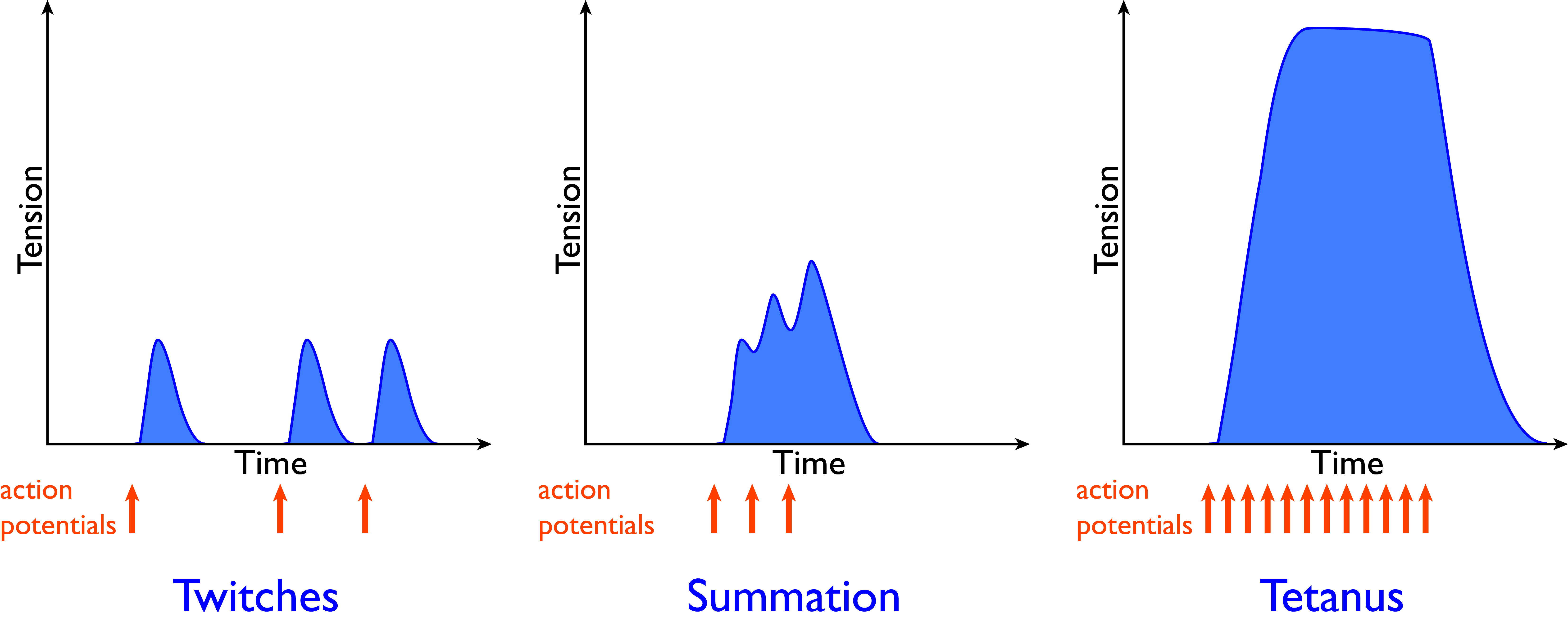 Figure 7.11 Twitch, summation, and tetanic contraction. In striated muscle, action potentials are an all-or-nothing response, but the force of contraction can be varied by varying the frequency of action potentials. CC0 Public Domain, 2017.
Figure 7.11 Twitch, summation, and tetanic contraction. In striated muscle, action potentials are an all-or-nothing response, but the force of contraction can be varied by varying the frequency of action potentials. CC0 Public Domain, 2017.
During locomotor activity, muscle contraction generates coordinated changes in length and tension in different muscles.
7.2.5 The Energy Supply
Muscle contraction requires energy in the form of ATP. Can you remember the two roles of ATP in the myosin ATPase cycle?[4] In addition to the hydrolysis of ATP by myosin, pumping calcium and sodium ions out of the myoplasm uses substantial amounts of ATP. The calcium pump that moves Ca2+ ions back into the sarcoplasmic reticulum accounts for as much as a third of the ATP hydrolysis in contracting muscle.
Total ATP hydrolysis in contracting muscle can be as much as 400 mM ATP per minute. Nevertheless, the amount of ATP in muscle cells stays almost constant, around 2 to 4 mM. How is this amazing feat of homeostasis possible? You’d think that this very limited amount of ATP would be used up in a fraction of a second during contraction. But it isn’t, because muscle cells have three metabolic pathways to restore used ATP by transferring phosphate to ADP: One pathway that rapidly regenerates ATP from a pool of high-energy phosphate, a second pathway that creates an oxygen debt by extracting some energy from glucose without using oxygen, and a third pathway that makes full use of oxygen to get the maximum amount of energy from glucose and fatty acids:
- Creatine phosphate as a pool of high-energy phosphate to transfer to ADP. Resting muscle cells make creatine phosphate using the enzyme creatine kinase, which phosphorylates creatine from ATP in a Mg2+-dependent, reversible reaction. Because of the reversibility of the creatine kinase reaction, creatine phosphate donates phosphate to ADP very rapidly. However, the total amount of energy provided is limited, and can only provide enough ATP for roughly the first thirty seconds to one minute of sustained contraction.
- Anaerobic glycolysis, or more accurately lactate fermentation, through which muscle cells obtain limited amounts of ATP from glycolysis of glucose without using oxygen. This pathway produces only 2 molecules of ATP per glucose molecule, and generates acid. If you sprint as fast as you can, acidification of muscle cells is the main reason why your muscles can only provide a third of the power five minutes into your sprint, compared with the start of your sprint. The lactate produced by fermentation represents an oxygen debt; Lactate buildup happens with sustained heavy exercise, and is one of a few reasons why it takes some time for you to catch your breath after you stop a strenuous activity.
- Aerobic oxidative phosphorylation (Figure…..) typically takes a couple of minutes to ramp up in muscle, but supplies approximately 30 molecules of ATP per glucose molecule, directly using oxygen. Aerobic oxidative phosphorylation in muscle can also make efficient use of fatty acids as an energy source to regenerate ATP. During sustained moderate activity, oxidation of fatty acids accounts for half or more of a muscle’s energy supply. Since mitochondria carry out oxidative phosphorylation, the rate of oxidative phosphorylation is limited by the number of mitochondria in muscle.
Notice that all three pathways serve to buffer the ATP concentration in the myoplasm such that the ATP level stays constant, regardless of how rapidly or slowly the muscle is using ATP. In any given muscle cell at any given time, all three metabolic pathways are operating, but the proportions vary. Typically, during a sudden burst of strenuous skeletal muscle activity, the muscle fibres use creatine phosphate first while shifting to anaerobic glycolysis, then use both anaerobic glycolysis and aerobic oxidative phosphorylation, and then later rely mostly on aerobic oxidative phosphorylation during prolonged endurance exercise.
Have you ever wondered about the difference between dark meat and light meat in chicken and turkey? The difference is due to which types of muscle fibre predominate in each muscle of the body.
As you might imagine, different types of skeletal muscle fibre are adapted to different needs. You could imagine one type of fibre that’s adapted to sustain a contraction for a long time without fatigue, and a different type of fibre that’s adapted to contract as rapidly as possible. Take a moment to think about which metabolic features would be different in different types of muscle fibre.
Indeed, there are many different types of skeletal muscle fibre. However, the vast majority fall into just three categories. Based on the main metabolic pathway that muscle cells use to obtain energy, two main types of muscle fibres can be recognized, glycolytic fibres and oxidative fibres. Oxidative fibres can be further classified into two main types based on contraction speed, for a total of three main types of skeletal muscle fibre.
Glycolytic fibres are characterized by having a high concentration of glycolytic enzymes and large stores of glycogen. Their myosin is usually a variant that has a very fast ATPase cycle. Thus, glycolytic fibres are adapted to generate a maximum amount of mechanical power quickly. They have limited capacity to use oxygen because they have relatively few mitochondria, are irrigated by few blood vessels, and contain little of the oxygen-binding protein myoglobin. Because these fibres depend on aerobic glycolysis, the synthesis of ATP is fast, but not sustainable. Thus, although glycolytic fibres are able to produce a lot of force quickly, they fatigue rapidly.
Oxidative fibres have a large number of mitochondria with high capacity for oxidative phosphorylation. Because of the high use of oxygen, oxidative fibres tend to be are highly vascularized (surrounded by many capillary blood vessels), and contain large amounts of the oxygen-binding protein myoglobin as an intracellular reservoir of oxygen. Two forms of oxidative fibres are found in muscle: fast oxidative fibres and slow oxidative fibres.
Fast-oxidative fibres contain myosin variants that have a fast ATPase cycle. Muscles with fast-oxidative fibres are suited for rapid actions, such as the rapid trilling sounds made by the throat muscles in songbirds or the clicking sounds of a rattlesnake’s shaking tail. Both fast oxidative fibres and glycolytic fibres are also known as fast twitch fibres.
Slow-oxidative fibres, also known as slow twitch fibres, contain myosin variants that have a slow ATPase cycle. Slow-oxidative fibres have relatively a low concentration of glycolytic enzymes and low stores of glycogen. Slow-oxidative fibres are resistant to fatigue and are capable of producing repeated contraction due to their capacity for producing large amounts of ATP.
Any one skeletal muscle will contain a mix of different fibre types, but the proportion of each fibre type in in the cross-sectional area of a given muscle varies depending on how the muscle is used. Remember the distinction between dark meat and light meat? The difference in muscle colour is due to a difference in the amount of myoglobin. Chickens and turkeys spend much of their time walking and running, and only fly in short bursts. Accordingly, their leg muscles are dark – rich in myoglobin – with many oxidative fibres, while their breast muscles are light – poor in myoglobin – with many glycolytic fibres.
The type of athletic training affects the relative proportion of fibre types in a muscle. For example, the leg muscles of marathon runners become rich in slow-oxidative fibre.
7.3 Smooth Muscle
Smooth muscles have a variety of different functions, therefore they show a variety of different structural and functional features and mechanisms of control. Depending on their function, smooth muscles can contract spontaneously, respond to neurotransmitters of the autonomic nervous system, respond to hormones and paracrine regulators, or respond to stretch signals originated in the same organ. In contrast to skeletal and cardiac muscle, smooth muscle does not show striations. Compared with striated muscle, smooth muscle fibres are more elastic and stretchable, and have a stronger stress-relaxation response. These features permit hollow organs to stretch when filled, but still are able to contract to empty the organ when stimulated. In the urinary system, smooth muscle allows the bladder to expand as it is filled while providing enough tension for urination. In the gastrointestinal tract, smooth muscle maintains tone when hollow organs empty, and shrink by synchronized, slow, and steady contractions that propel food through the gut.
The structural units of the smooth muscle are small, spindle-shaped cells with single nucleus, termed smooth muscle myocytes. Like striated muscle, smooth muscle myocytes can tense and relax. Two different structures composed of smooth muscles can be distinguished in vertebrates, single-unit muscle and multi-unit muscle.
Single-unit smooth muscle is characterized by a large number of gap junctions that connect the myocytes with each other, and couple them electrically. Electrical coupling through gap junctions allows the myocytes to contract together in a co-ordinated way. When smooth muscle contracts regularly without neural input, contraction is said to be myogenic instead of neurogenic. Myogenic contraction is due to a group of mesoderm-derived intramuscular cells, termed interstitial cells of Cajal (ICC-IM), or pacesetters that work as pacemakers. ICC-IM cells are distributed throughout the gastrointestinal tract generating action potentials that lead to smooth muscle cell contraction. They contract rhythmically the walls of most visceral organs, even in the absence of neural input.
Multi-unit smooth muscle in general does not have gap junctions; therefore, contraction is limited to a single innervated myocyte. Unlike single-unit muscle, multi-unit smooth muscle are not sensitive to stretch, but they are extensively innervated by the autonomic nervous system that controls contractions in fine gradual responses, much like the response of the motor unit in skeletal muscle. Multi-unit smooth muscles contract the eye pupil, hair follicles, feather erectors, large arteries, and respiratory airways.
7.3.1 Coupling of Contraction to Excitation
Although the mechanism of contraction is similar between skeletal and smooth muscle, the regulation of contraction presents several differences. Unlike skeletal muscle fibres, smooth muscle myocytes do not have motor end-plates. Instead, they have swollen regions of the axons known as varicosities (Figure….), through which a single motor neuron is able to innervate multiple cells, thereby causing them to contract at the same time. They respond to spontaneous signals such as those from the ICC-IM pacemakers, stretch signals, and signals from the ANS that modulate rate and strength of contraction.
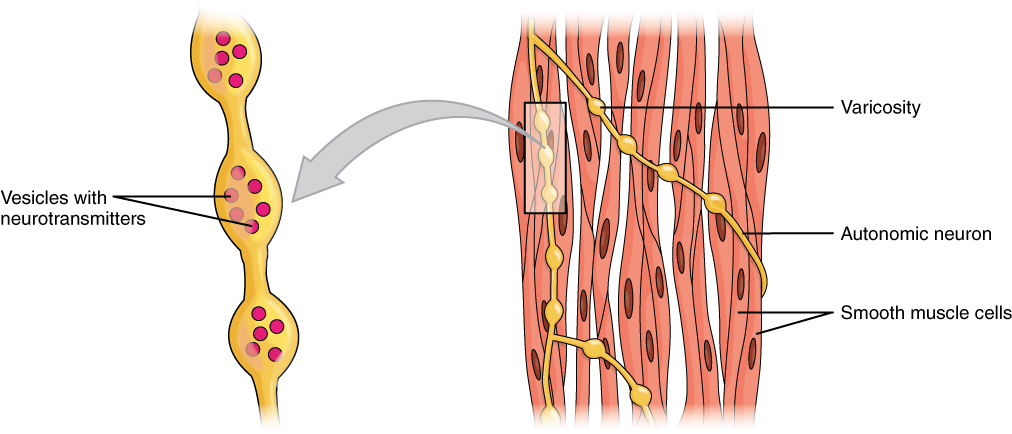 Figure 7.12 Smooth muscle is innervated by axons of the autonomic nervous system. Varicosities in the axons release the neurotransmitter. Source: Anatomy & Physiology, Rice University, download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.25 CC BY 4.0, 2016.
Figure 7.12 Smooth muscle is innervated by axons of the autonomic nervous system. Varicosities in the axons release the neurotransmitter. Source: Anatomy & Physiology, Rice University, download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.25 CC BY 4.0, 2016.
The myofilaments of the smooth muscle are not organized into sarcomeres. Smooth muscle myocytes have a rudimentary SR, lack T-tubules, and are usually arranged in sheets within the muscle tissue. They contain myosin, actin and tropomyosin, but unlike the striated muscle, smooth muscle does not contain troponin.
Calcium ions are supplied by the SR in the fibres and by sequestration from the extracellular fluid through membrane indentations called caveolae . In contrast to skeletal muscle fibres, the action potentials in smooth muscle myocytes are generated by influx of Ca2+ instead of Na+; and like the skeletal muscle fibre, cytosolic Ca2+ ions are essential for crossbridge cycling. Ca2+ ions enter into the cytoplasm of smooth muscle myocytes from the extracellular pool and from the sarcoplasmic reticulum stores. In the cytoplasm Ca2+ binds to the Ca2+-binding protein calmodulin, which activates a myosin light-chain kinase, the Ca2+-calmodulin-myosin-light-chain kinase that phosphorylates myosin, initiating contraction by activating the myosin ATPase. Phosphate removal by myosin light chain phosphatase, terminates crossbridge cycling, and leaves the smooth muscle in a latch-state.
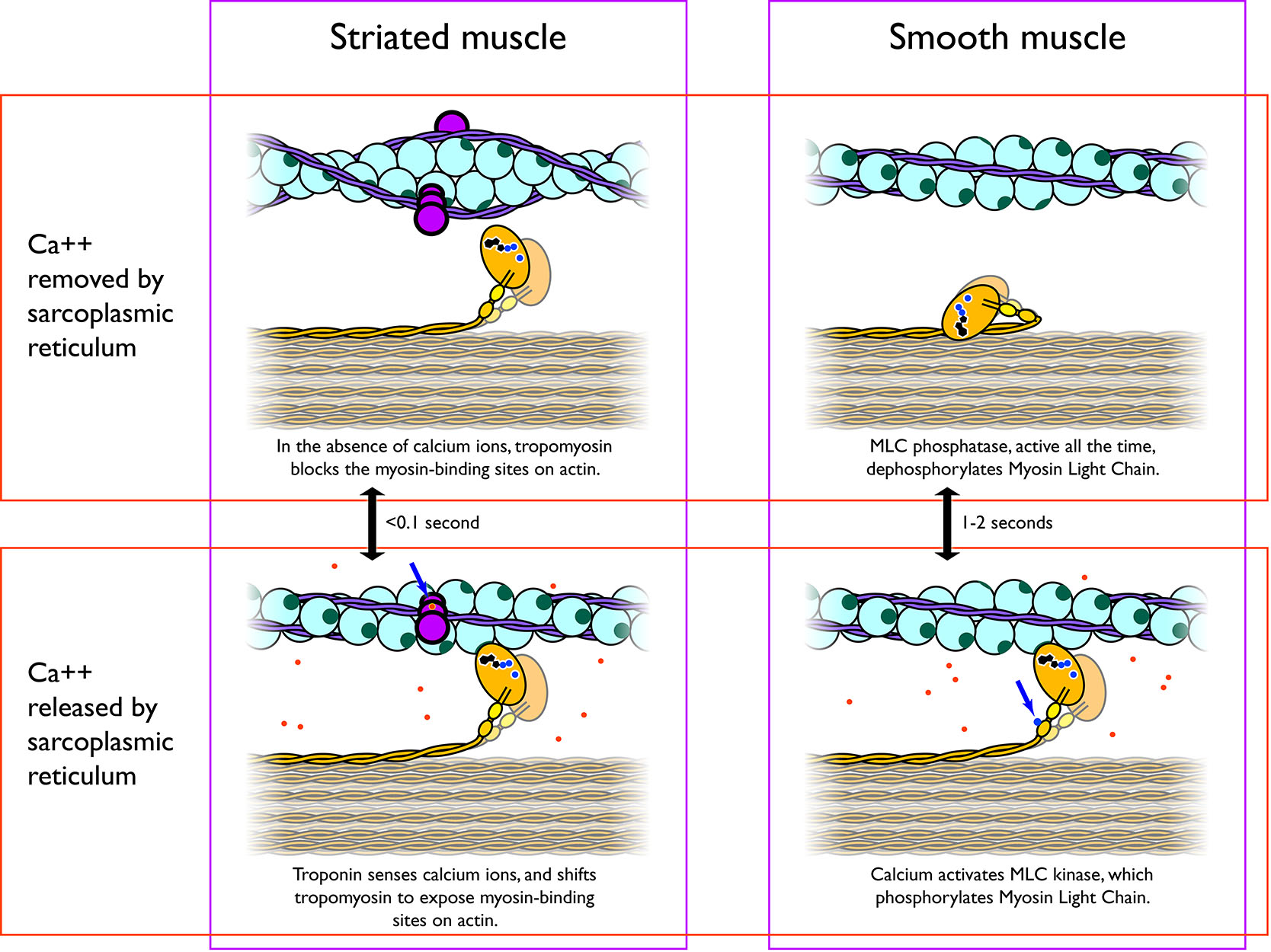 Figure 7.13 Calcium ions released from the sarcoplasmic reticulum into the sarcoplasm are the signal for muscle contraction. CC0 Public Domain, 2017.
Figure 7.13 Calcium ions released from the sarcoplasmic reticulum into the sarcoplasm are the signal for muscle contraction. CC0 Public Domain, 2017.
7.3.2 Neuromodulation of Smooth Muscle Activity
Although contraction of the single-unit smooth is myogenic, its activity is modulated by the neurotransmitters of the autonomic nervous system and by multiple other inputs, such as hormones, chemical composition of the local intercellular fluids, and stretch of the organ. Like the postsynaptic membrane of a neuron, input signals can be either excitatory or inhibitory on the smooth muscle sarcolemma. Unlike an axon’s plasma membrane, responses of the smooth muscle cell membrane can be graded.
Smooth muscles are innervated by both the parasympathetic and sympathetic divisions of the ANS. Postganglionic nerve fibres of parasympathetic nervous system release ACh that bind to the metabotropic G-protein coupled muscarinic acetylcholine receptors (mAChRs) located on the myocyte’s cell membrane. Conversely, postganglionic nerve fibres of the sympathetic nervous system release the neurotransmitters epinephrine and norepinephrine, which bind to adrenergic receptors that are also metabotropic. The exact effects of the ANS on the smooth muscle depend on the specific characteristics of the receptor activated – both parasympathetic input and sympathetic input can be either excitatory (contractile) or inhibitory (relaxing).
Neurotransmitters of the sympathetic division of the ANS, such as norepinephrine enhances contraction of most vascular smooth muscle by acting on α-adrenergic receptors, but produce relaxation of airway (bronchiolar) smooth muscle by acting on β2 adrenergic receptors. Thus, the type of response, whether is excitatory or inhibitory, depends not only on the neurotransmitter, but on the membrane receptors present in a particular tissue and on the intracellular signalling mechanisms those receptors activate (Chapter….).
Smooth muscles also respond to local factors, including paracrine signals, acidity, O2 and CO2 levels, osmolarity, and the ion composition of the extracellular fluid. All of these factors alter smooth muscle contraction. These regulators provide finer control on smooth muscle contraction because they respond to immediate signals from the local extracellular environment, independent of the long-distance signals emerging from the ANS and the endocrine system. One example of a local paracrine factor is nitric oxide, which signals smooth muscle to relax. Nitric oxide is particularly important in telling the smooth muscle in the walls of blood vessels to relax when greater blood flow is needed locally.
Some smooth muscles also respond to stretching. Tension in the smooth muscle cell sarcolemma opens mechanosensitive ion channels, leading to membrane depolarization, resulting in contraction that opposes the forces acting to stretch the muscle.
Smooth muscles also respond to hormones. Hormones can have an immediate effect on the force of smooth muscle contraction, but can also have slower, longer-term effects on smooth muscle structure. For example, hormones can tell smooth muscle to grow in size (hypertrophy). Often, hormone-stimulated growth of smooth muscle is due to growth in the size of each cell, but hormones can also cause the muscle cells to divide to produce more cells (hyperplasia). Examples of these processes can be seen in the uterus: At puberty, increased levels of estrogens (a type of steroid hormone) trigger growth of the uterus. The myometrium, the smooth muscle layer of the uterus, increases in size both through an increase in both smooth muscle fibre (cell) size and smooth muscle fibre number. During most of pregnancy, the steroid hormone progesterone limits the number of gap junctions between myometrium fibres. At the very end of pregnancy, the level of progesterone drops, resulting in a dramatic increase in the number of gap junctions between myometrium fibres. The increased electrical coupling of cells through gap junctions allows the uterus to respond to the hormone oxytocin – released during childbirth – a single-unit smooth muscle. Thus, the uterus becomes adapted to produce the coordinated contractions of childbirth.
7.4 Summary
7.5 Suggested Readings
7.6 Glossary
A-band – In striated muscle, the A band is the portion of the sarcomere that contains thick filaments. In thin sections of muscle prepared for microscopy, the A band stains darker than the I band.
α-motor neuron – Neuron whose cell body is in the gray matter of the spinal cord and brainstem, and has a large, myelinated axon that extends to neuromuscular junctions on skeletal muscle. α-motor neurons are the main type of neuron that triggers voluntary muscle contraction.
ACh – Acetylcholine.
acetylcholine – Acetylcholine is a small molecule that acts as a neurotransmitter at the neuromuscular junction and some other parts of the nervous system.
actin – Actin is a protein that polymerises to form the thin filaments of the cytoskeleton in all eukaryotc cells. In muscle, actin filaments are the thin filaments of the sarcomere.
aerobic oxidative phosphorylation – Use of molecular oxygen to get energy to make ATP from ADP and phosphate. Mitochondria are where aerobic oxidative phosphorylation takes place.
anaerobic glycolysis – Breakdown of glucose to make ATP from ADP and phosphate without using oxygen. Anaerobic glycolysis is faster, but much less efficient than aerobic oxidative phosphorylation.
calmodulin – Calmodulin is a small protein that binds to calcium ions.
calsequestrin – Calsequestrin is a calcium-binding protein in the lumen of the sarcoplasmic reticulum; It helps the sarcoplasmic reticulum to store a large amount of calcium.
concentric contraction – Muscle contraction where muscle length decreases because tension exceeds load.
creatine phosphate – Creatine phosphate is a small molecule that serves as a short-term store of high-energy phosphate to regenerate ATP from ADP during muscle contraction.
DHPR – Dihydropyridine receptor
dihydropyridine receptor – Also known as the Voltage-gated L-type Ca2+ Channel, the DHPR is concentrated in the T-tubule membrane, where it responds to membrane depolarisation by releasing Ca++ into the sarcoplasm, and also directly activating the ryanodine receptor.
dimer – An object that consists of two parts.
eccentric contraction – Muscle contraction where muscle length increases because load exceeds tension.
fast oxidative fibre – Fast twitch muscle fibre that is rich in mitochondria and relies mostly on aerobic oxidative phosphorylation.
fast twitch fibre – Muscle fibre whose myosin has a rapid ATPase cycle. Fast twitch fibres contract rapidly, but also fatigue rapidly.
glycolytic fibre – Muscle fibre rich in glycogen and in glycolytic enzymes. Glycolytic fibres are specialised to rely on anaerobic glycolysis for ATP production.
H-band – A lighter band in the middle of the A band. It is lighter because it lacks thin filaments.
hyperplasia – Tissue growth by cell division.
hypertrophy – Tissue growth by growth in cell size.
I-band – In striated muscle, the I band is the portion of the sarcomere that lacks thick filaments. In thin sections of muscle prepared for microscopy, the I band stains lighter than the A band.
ICC-IM – interstitial cell of Cajal
isometric contraction – Muscle contraction where musce length stays the same.
isotonic contraction – Muscle contraction where tension remains constant.
interstitial cell of Cajal – Pacemaker cell of the heart.
lactate fermentation – Production of lactic acid as a waste product of anaerobic glycolysis.
load – The force exerted on a muscle. Contrast with tension.
M-line – The line where the thick filaments (myosin filaments) of the sarcomere are tethered together.
motor end-plate – Specialised area of the sarcolemma packed with acetylcholine receptors, at the neuromuscular junction.
multi-unit muscle – Smooth muscle that has few gap junctions. Contraction is neurogenic, because every muscle cell needs a direct signal from a neuron. Contrast with single-unit muscle.
myoblast – Myoblasts are muscle progenitor cells that will develop into myocytes.
myocyte – Muscle cell, specifically a cell that’s specialised for producing force and contracting.
myofilament – Filament of the sarcomere, either a thick filament (myosin), a thin filament (actin), or an elastic filament (titin).
myofibril – A rodlike organelle consisting of a chain of sarcomeres.
myogenic contraction – Muscle contraction where the signal that triggers contraction starts from the muscle itself. Contrast with neurogenic contraction.
myoglobin – Myoglobin is an oxygen-binding protein in the sarcoplasm of muscle cells.
myosin – Myosins are motor prteins that produce a sliding force against actin filaments.
nebulin – A long protein, much longer than tropomyosin, that is a part of the thin filaments of sarcomeres.
neurogenic contraction – Muscle contraction where the signal that triggers contraction starts from the nervous system. Contrast with myogenic contraction.
neuromuscular junction – The contact between a neuron and a muscle cell, the neuromuscular junction is a special kind of chemical synapse.
NMJ – Neuromuscular junction
oxidative fibre – Muscle fibre that is rich in mitochondria and is specialised to use aerobic oxidative phosphorylation as its main source of ATP.
power stroke – Force-producing change in the shape of the myosin head, swinging the lever arm 60°. In the absence of load, this moves the thick filament 8 nm relative to the thin filament in muscle.
relaxation – The loss of tension in muscle when the cross-bridges between filaments stop forming as the sarcoplasmic reticulum removes calcium from the sarcoplasm.
ryanodine receptor – Also known as the SR-Ca2+ release channel, the ryanodine receptor is the endoplasmic reticulum membrane protein responsible for calcium-induced calcium release. It releases calcium ions into the sarcoplasm from the sarcoplasmic reticulum in response to action potentials in the sarcolemma (the muscle cell plasma membrane).
RyR – Ryanodine receptor
sarcolemma – The plasma membrane (cell membrane) of the muscle cell.
sarcomere – The basic unit of actin-myosin contractile machinery in striated muscle. By convention, one sarcomere is defined as the segment of myofibril from one Z-disc to the next Z-disc.
sarcoplasm – The cytoplasm of the muscle cell.
sarcoplasmic reticulum – The endoplasmic reticulum of muscle cells. The SR stores, releases, and retrieves the calcium ions that signal contraction.
single-unit muscle – Smooth muscle that has a lot of gap junctions, allowing action potentials to spread from one cell to many others. Contrast with multi-unit muscle.
sliding filament – Sliding of thick filaments and thin filaments past each other during contraction.
slow oxidative fibre – Slow twitch muscle fibre that is rich in mitochondria and relies mostly on aerobic oxidative phosphorylation.
slow twitch fibre – Muscle fibre whose myosin has a slow ATPase cycle. Slow twitch fibres contract slowly, but also fatigue slowly, and can maintain constant tension for a long time.
SR – sarcoplasmic reticulum
SR-Ca2+ release channel – Also called the ryanodine receptor
summation – Summation is where action potentials or stimuli are sufficiently close together in time to result in a stronger muscular contraction than a single twitch.
T-tubule – transverse tubule
tension – The force exerted by a muscle. Contrast with load.
tetanus – A tetanic contaction is where many action potentials or stimuli are sufficiently close together in time to result in continuously maximal muscular contraction.
thick filament – Myosin filament in muscle.
thin filament – Actin filament.
titin – The largest protein in the human body, titin connects the M line to the Z disks in striated muscle, and gives muscle its elasticity. The long, elastic molecule of titin keeps the thick filaments of the sarcomere aligned during muscle stretching and contraction.
transverse tubule – Membrane tubule leading from plasma membrane of the muscle cell to the sarcomeres, coming in close contact with the sarcoplasmic reticulum. Thus, T tubules allow action potentials to reach the interior of muscle cells for more rapid signalling to the sarcoplasmic reticulum.
tropomyosin – A long, thin protein that lines an actin filament, stabilising it. Each tropomyosin molecule is a single, long alpha-helix that forms a coiled-coil dimer. In the sarcomeres of striated muscle, tropomyosin controls contraction by blocking myosin from binding to actin when calcium levels are low in the sarcoplasm.
troponin – A group of three proteins which together allow sarcomeres of striated muscle to sense calcium. The troponin complex responds to calcium by shifting the position of tropomyosin to allow myosin to access the thin filament.
twitch – The force or contraction triggered by a single action potential.
voltage-gated L-type Ca2+ channel – Also known as the dihydropyridine receptor.
Z-line – The line formed by the Z-disc, which tethers the thin filaments (actin filaments) of the sarcomere.
- The sinoatrial node consists of a group of specialized cardiomyocytes that generate electrical impulses at rates higher than the rest of the cardiac cells. They set up the cardiac rhythm of contraction, working as pacemakers. ↵
- Myocytes of the pectoral muscles of chickens contain very little or no myoglobin. ↵
- Saltatory = “jumping.” Remember from Chapter 5 that long motor neurons are myelinated for more efficient, faster transmission of action potentials. ↵
- Releasing the myosin head from actin, and cocking the myosin head. ↵

