12. Metabolism, and Energy Balance
Kim Cross, with contributions from Daniel Schott
12.1 Introduction: Metabolism and Energy
12.2 Organic Nutrients as Energy Sources
12.3 Control of Appetite
12.4 Absorptive and Post-Absorptive States
12.5 Metabolic Rate, and Energy Storage
12.6 Control of Body Temperature
12.7 Summary
12.8 Glossary
Feast or famine
What’s the longest you’ve gone without food? What’s the longest your classmates have gone without food? In Canada today, most young adults have never experienced starvation. But many young Canadians have parents, grandparents, or great-grandparents who lived through a famine at some point in their lives. And around the globe, hundreds of millions of people do not have enough to eat.
In the more distant past, our ancestors might not have experienced an actual famine very often, but food shortages would have been common. They had a limited ability to store food, and in places that have a cold season or a dry season, this meant that there was little food for part of each year. Our prehistoric ancestors often had to walk long distances to find food. Hunting and gathering food was often hard physical work. So, for much of our recent evolutionary history, the pattern of our lives was alternating depletion and reload of energy.
The human body has evolved to efficiently store away surplus energy and be frugal in using it: Those individuals who could put on a substantial extra weight as fat during times of plenty, and keep that weight afterwards, were more likely to survive and have more children. Thus, the human body is adapted for cycles of feast and famine.
Today, we just load up on nutrients nonstop, without expending much energy. The modern industrialised lifestyle has led to an epidemic ofmetabolic disorders such as diabetes and obesity.

Figure 12.1 Body image, 25 000 BCE and 1955. Society’s concepts of beauty have veered from one extreme to another, both of them unhealthy. Through most of human history, obesity was a sign of high social status in many cultures around the world. Only the wealthiest could afford to eat every day. Only ordinary people did hard physical labour. Today, waistlines in most countries are expanding. In highly developed countries, only the wealthy can afford to stay physically active and eat a healthy diet. So being skinny became fashionable. Left: photo, Matthias Kabel CC BY 2.5; sculptor unknown, 25 000 BCE https://commons.wikimedia.org/wiki/File:Venus_von_Willendorf_01.jpg; Right: photo, Dimitry B. CC BY 2.0; sculptor Alberto Giacometti, ca. 1955 https://www.flickr.com/photos/ru_boff/7189057682.
12.1 Introduction: Metabolism and Energy
The need for energy is one of the main principles of life, as you’ll remember from Chapters 8 and 10 on digestion and respiration.
Thus, while animals need nutrients as the basic building blocks of the body, that isn’t the only all-important role of nutrients: Nutrients, in combination with oxygen (O2)1, supply the energy needed to maintain the complex organisation and homeostasis of the body.
The body obtains energy by oxidising nutrients. This set of chemical reactions is called cellular respiration. You’ll often hear cellular respiration being called “aerobic respiration”, because cellular respiration needs O2 in 99.999% of animal species – almost all animals. For example, the use of glucose and O2 for energy can be summarised by the formula:
C6H12O6 + 6 O2 6 CO2 + 6 H2O + energy
Of course, this formula is a gross oversimplification of the many chemical reactions that manipulate nutrients. Keep in mind that all organic nutrients – not only glucose – can be used as sources of energy.
This chapter will discuss homeostatic control of the three main organic nutrients obtained in the diet: carbohydrates, lipids, and proteins. This chapter will also explore the roles of these nutrients in metabolism, and develop a full picture of energy use in an animal’s body by exploring metabolic rates, thermoregulation, and weight gain or loss.
A crash course on energy
So far, we’ve avoided defining the word “energy”. In the context of biology, you can think of energy (Greek en: “in”, and érgon, “work”) as the capacity to do mechanical work, do chemical work, and generate heat. This isn’t a complete definition of the word “energy”, or an entirely accurate one. But it’s a good enough definition to understand how the animal body works.
By definition, energy is always conserved: Physicists define “energy” as a quantity that does not change no matter what happens2. Energy is a fascinating and sometimes confusing topic. But the important thing to remember is that energy cannot be created and cannot be destroyed; it can only change form or be transferred to another object.
Suppose you throw a ball straight up in the air. When the ball leaves your hand, it has a lot of kinetic energy. Kinetic energy is the energy of motion. As the ball goes upwards, it slows down due to gravity. Its kinetic energy gets converted into gravitational potential energy. Eventually, the ball stops moving. All of its kinetic energy has been converted into gravitational potential energy. Then, the ball reverses direction, and picks up speed as it falls downwards. Its gravitational potential energy is converted back into kinetic energy.

Figure 12.2 Potential energy, and kinetic energy. A living cell stores chemical potential energy for later use to do the work required to maintain life. “The Toboggan Party”, Rideau Hall composite photo: William James Topley / Library and Archives Canada http://collectionscanada.gc.ca public domain, ca. 1874.
Potential energy can be stored for later use. Imagine pulling a toboggan up a hill. By going uphill, you’re building up gravitational potential energy. This is stored energy as long as you stay at the top of the hill. Living cells store energy too, in the form of chemical potential energy. The main long-term energy store in animals is fat, mostly as triglycerides. Another important energy store in animals is glycogen, a polymer of the sugar glucose. We’ll return to fat and glycogen later in this chapter. Back to tobogganning: To release the stored energy, you give the toboggan a little shove – if you’re familiar with enzymology from previous biology classes, you’ll recognise this as the activation energy for a chemical reaction – and the toboggan will pick up speed going downhill.
While energy can neither be created nor destroyed, in a complex system such as an animal cell not all of the energy is available to do mechanical or chemical work; Whenever an animal’s body expends energy, some of the energy is lost as heat.
For example, consider the energy efficiency of muscle. A mammalian skeletal muscle typically achieves a maximum efficiency of 30%: At best, the muscle converts 30% of the chemical potential energy into mechanical work, while the muscle loses the remaining 70% as heat. How does this compare with the fuel efficiency of a car engine? Gasoline-burning internal combustion engines in cars actually have the same range of fuel efficiencies as skeletal muscle: around 20-35%.
For a chemical reaction in a living cell, the maximum amount of energy available to do mechanical or chemical work can be represented by the Gibbs energy, in the formula
∆G = ∆H – T∆S
where
- ∆G is the Gibbs energy;
- ∆H is the change in enthalpy, which includes heat released or absorbed by the reaction;
- T is the absolute temperature (in Kelvins); and
- ∆S is the change in entropy.
The Gibbs energy of a chemical reaction is closely related to the equilibrium constant for that chemical reaction. At chemical equilibrium, where the rate of the forward reaction equals the rate of the reverse reaction, ∆G is zero. If the forward reaction is energetically favoured over the reverse reaction, ∆G is negative. If the reverse reaction is energetically favoured over the forward reaction, ∆G is positive.
Entropy is an important concept for understanding why living things need energy. A slightly technical definition of entropy: Entropy is the amount of information needed to describe the microscopic internal state of a system. A less technical definition: Entropy is the amount of microscopic disorder in a system. According to the Second Law of Thermodynamics, time always flows in the direction of increasing entropy. If you’ve ever had a desk, a bedroom, a garage, or even a house, you’ll be familiar with the idea that clutter always increases with the passage of time!
If total entropy never goes down with time, how can living things possibly maintain the complex organisation of their bodies and cells? How they deal with ever-increasing disorder?
They do it by increasing the entropy of their environment, releasing the heat generated by the chemical reactions inside the cell. You’re already familiar with this idea: To organise and clean up a cluttered desk takes a lot of energy, and this work invariably results in an increase of clutter somewhere else.
Let’s introduce a few more terms. The sum total of the thousands of complex chemical reactions inside cells is called metabolism, each biochemical reaction commonly called a metabolic reaction. There are several pairs of terms that describe categories of metabolic reactions.
For example, some reactions may be catabolic – degrading larger molecules into simpler, smaller molecules, while other reactions are anabolic – building more complex molecules from simpler ones. See Figure 12.3 (next page) for a summary of catabolic and anabolic pathways that process nutrients from food.
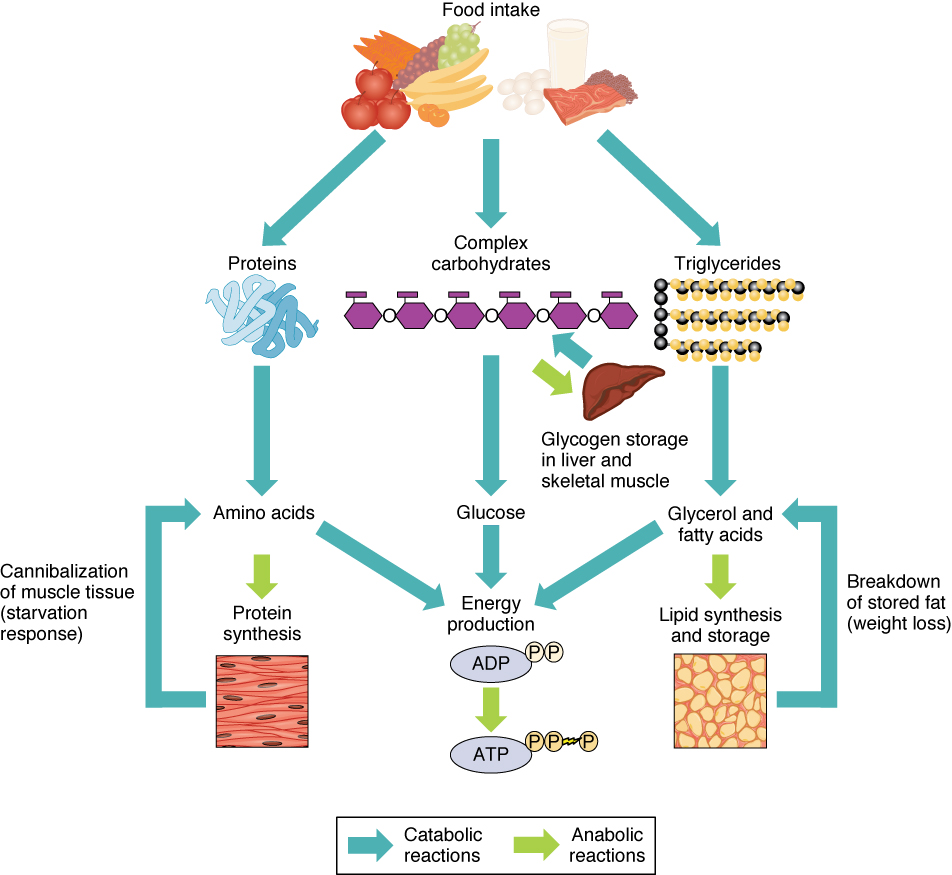
Figure 12.3 The main catabolic and anabolic fates of food in the human body. Source: Anatomy and Physiology, Rice University. Download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@10.1. CC BY 4.0, 2018.
Many of the same reactions may be called oxidative – if molecules lose electrons, or reductive – if molecules gain electrons. In biology, you normally see transfer of electrons from one molecule to another, such that each reaction is both oxidation and reduction at the same time. These reactions are called redox reactions because one molecule gets reduced while the other molecule gets oxidised.
Additionally, the reactions can be termed endergonic – when the reaction requires energy from a system to proceed (∆G is positive), or exergonic – when the reaction donates energy to a system (∆G is negative). In order to drive endergonic reactions in the forward direction, living things couple each endergonic reaction with an exergonic reaction, such that the total reaction, both combined, is exergonic.
ATP (adenosine triphosphate) hydrolysis is the exergonic reaction that enzymes most commonly use to drive an endergonic reaction forwards. You could say ATP is the energy currency of the cell. Other energy carrying molecules include GTP (guanosine triphosphate), NADH (nicotinamide adenine dinucleotide) and FADH2 (flavin adenine dinucleotide), but ATP is the most abundant, and is used in the greatest number of chemical reactions in the cytoplasm. ATP is the main energy-carrying product of cellular respiration, the main energy source for animals.
We may as well introduce one more pair of terms describing chemical reactions: Exothermic reactions have negative ∆H. In living systems, this almost always refers to the production of heat. Endothermic reactions have positive ∆H, typically absorbing heat. Most processes in living things are exothermic, but there is an important exception: Evaporation of water is a process that’s both strongly endothermic and exergonic (as long as relative humidity is low). As you’ll learn towards the end of this chapter, cooling by evaporation is important in many land animals.
ATP, the energy currency of the cell
You may recall from past biology classes: cellular respiration is often broken into three or four major pathways. Glycolysis, the breaking of glucose to form pyruvate in the cytosol, generates a small amount of the energy-carrying molecules ATP and NADH. The remaining steps happen in the mitochondria of the cell. In animals and all other eukaryotes, mitochondria are the site of cellular respiration in the cell. The modification of pyruvate into acetyl-CoA releases a little more energy as NADH. The citric acid cycle then degrades acetyl-CoA to release many forms of energy in the process (ATP, GTP, NADH, and FADH2). Finally, oxidative phosphorylation generates large amounts of ATP. Public service announcement: cellular respiration is often taught as the total breakdown of glucose through these pathways. However, a range of different organic molecules can serve as fuel for cellular respiration, see Figure 12.3.
Additionally, cells of most animals are able to get a little bit of energy from glucose without using oxygen. Animal cells typically accomplish this feat by a metabolic process called lactic acid fermentation. You’ll already have heard about lactic acid fermentation in Chapter 7, on muscle. To review, glucose enters the aerobic respiration pathway by way of glycolysis, generating two molecules of pyruvate per molecule of glucose, with a net gain of two molecules of ATP. Normally, mitochondria burn the pyruvate to generate more than ten times as much ATP in the electron transport chain, using O2 as the electron acceptor. If the cell needs to avoid using oxygen, pyruvate can instead be reduced to produce lactate. Lactic acid fermentation allows the cell to use the small amount of ATP generated by glycolysis, without using O2. There is a limit to how much lactic acid buildup animal tissues can tolerate, because the accumulation of excess acid disrupts the homeostasis of pH. The lactate isn’t a waste product – it’s still a valuable energy source when and where oxygen is available. Active skeletal muscle typically produces a lot of lactate due to lactic acid fermentation (see Chapter 7, on muscle), and the excess lactate travels by way of the bloodstream to the liver, which burns some of the lactate as an energy source and converts the rest back into glucose. This is a well-known metabolic cycle in the animal body (see Figure 12.4, next page). One reason you’re out of breath for a while after vigorous exercise: If lactate builds up in the body faster than respiration can deliver oxygen, the lactate contributes to a temporary “oxygen debt”.
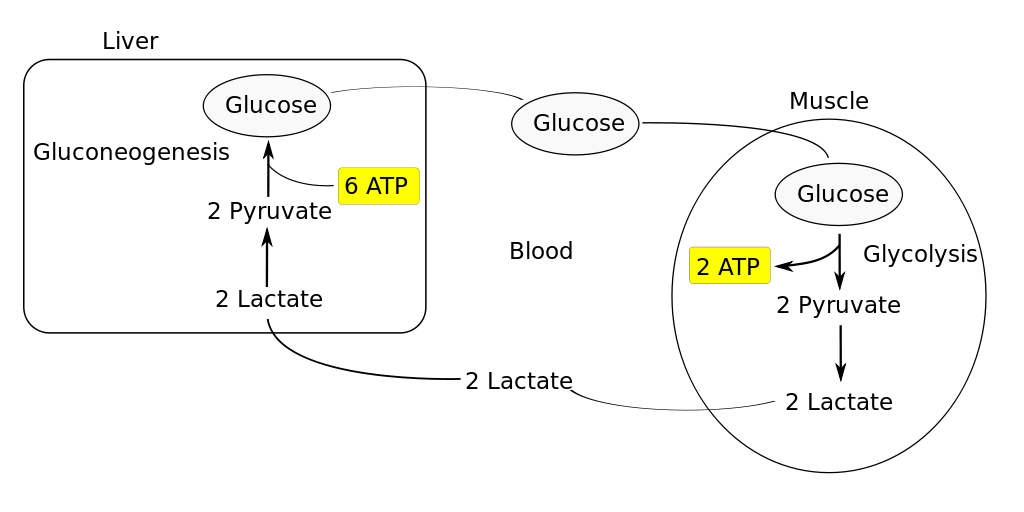
Figure 12.4 Lactic acid fermentation in skeletal muscle, and regeneration of glucose in the liver. Source: Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Cori_Cycle.SVG CC BY-SA 3.0, 2011.
12.2 Organic Nutrients as Energy Sources
The body can use most of the organic nutrients in the diet as sources of energy, but carbohydrates, lipids, and proteins are usually the most abundant energy sources in food. The exact order of use and preferred role of each nutrient varies from nutrient to nutrient and from animal to animal. All organics are obtained in the diet, all are digested into simpler molecules prior to absorption, and all can be converted into other products and energy by the body. Reviewing Chapter 8, carbohydrates are absorbed as monosaccharides like glucose, fructose and lactose. Lipids are absorbed as fatty acids and monoglycerides, while proteins are absorbed as amino acids. In terms of energy, the general order of nutrient use is: carbohydrate first, then lipids, and finally protein (amino acids).
Carbohydrates, commonly circulating as glucose, supply tremendous amounts of energy to the body, especially the nervous system. When required for energy immediately, carbohydrates can enter cellular respiration during glycolysis, or carbohydrate by-products enter the citric acid cycle. If there are excess carbohydrates in the body, they can be stored as glycogen or converted into triglycerides (lipids). Additionally, carbohydrates can be used as structural molecules in animals, for example, glycosaminoglycan in connective tissues.
Lipids, commonly circulating as fatty acids and monoglycerides, supply the second largest supply of energy to the body. If tissues other than the nervous system require energy, and glucose is not readily available, fatty acids and other lipid products will enter the citric acid cycle. If there isn’t enough glucose available to supply the nervous system with energy, the liver must convert lipids into ketone bodies (beta-hydroxybutyrate, acetoacetate, and acetone), which can then enter the citric acid cycle. The central nervous system (CNS) is different from most other tissues in the body: Despite using ten times as much energy per unit weight as the rest of the body, the brain and spinal cord don’t use fatty acids as their main energy source; Instead, glucose is the main energy source, ketone bodies when not enough glucose is available. It used to be thought that this is because fatty acids cannot cross the blood-brain barrier into the CNS, but researchers now believe it’s more likely because CNS neurons are very long-lived cells, and therefore sensitive to cumulative damage by byproducts of fatty acid oxidation.
However, compared to carbohydrates, lipids are often thought of as long-term stored energy and commonly stored as triglycerides in adipose tissue and the liver. Lipids play many other roles in the body including structural components of cellular membranes (phospholipids) and as signalling molecules (especially steroids).
Proteins are digested into amino acids, which circulate in the blood. Amino acids have the potential to supply large amounts of energy, yet they fall to the bottom of the list of energy molecules. Amino acids and their by-products can easily enter the citric acid cycle to supply fuel for the body. However, there are two main concerns around the metabolism of amino acids: their importance for building new proteins and the production of ammonia during their metabolism. The importance of amino acids during the creation of new proteins partially explains their limited use for energy. Proteins are cellular machines, produced via gene expression. In other words, proteins are they way your genes talk to the world. Proteins are responsible for most of the body’s structure and function. For example, proteins are membrane pumps and channels, enzymes, signalling molecules, antibodies, haemoglobin, and the main components of muscle and bone. If an animal’s body switches to using amino acids and proteins for energy, it is generally a survival mechanism. Finally, the metabolism of proteins and amino acids generates ammonia, a nitrogenous toxic waste. Many animals convert ammonia into to urea (mammals and sharks for example), while others convert ammonia into non-soluble forms like uric acid (some insects, reptiles and birds). Regardless, all nitrogen wastes must be removed from the body and all ammonia conversion pathways are fairly energy intensive, rather than energy yielding, processes.
12.3 Control of Appetite
The digestive system is the initial system involved in the homeostatic control of nutrients in an animal’s body. The rates of digestion and the amount of nutrients absorbed directly influences the levels of nutrients circulating in the blood. However, when does an animal eat? This sometimes depends on availability of food. How much does an animal eat? This can depend on the quality of the food (palatability or taste) and quantity of nutrients in the food. For example, if the taste of food is not agreeable, it is expelled from the mouth. There are many external factors contributing to the “when and how much” questions.
A review of Chapter 8 reveals the digestive system also regulates itself. If there is too much food in the intestinal system, the rates of swallowing and stomach movement slow down. This is regulation occurs via the enteric nervous system (ENS), and through local signalling molecules.
Additionally, there are many signalling pathways leading from the digestive system to the CNS to help regulate appetite (hunger) and satiety (feeling of being full). Studies have shown the hypothalamus to be very important in the regulation of feeding, with possible appetite centers and satiety centers directly controlling feeding levels. Besides the digestive system, there are other regions of the body, like adipose tissue, which will have some regulation over hunger and satiety. It should come as no surprise, there are many hormones released by the digestive system and adipose tissue to signal other components of the digestive system and the nervous system or hypothalamus. Three well-studied hormones regulating appetite and satiety are cholecystokinin (CCK), ghrelin and leptin.
CCK is released by the small intestine when food is present, especially when the food has high concentrations of fats and proteins. CCK has many targets in the body, the first being the pancreas and liver. CCK stimulates pancreatic enzyme production and bile release from the liver. Therefore, the initial actions of CCK would lead to an increase in circulating nutrients in the body. However, another major target of CCK is the hypothalamus. The result is a suppression of appetite, or an increase in satiety. Over time, this will decrease the levels of circulating nutrients.
Ghrelin is released by the stomach and small intestine when food has been absent for a short period. It also has many targets in the body. Ghrelin influences the brain and hypothalamus/anterior pituitary, resulting in increased appetite. Ghrelin also contributes to the feed-forward response during the cephalic phase of gastric control and may inhibit insulin production by the pancreas. All these actions would eventually lead to an increase in circulating nutrients.
Leptin is primarily released by adipose tissue, in response to increased levels of triglycerides in the adipose cells. When the amount of adipose tissue increases, so does the production of leptin. Increased amounts of fat in adipose tissue would suggest feeding has occurred for a great deal of time, and nutrient levels in the body are high. Would you expect leptin increases appetite or increases satiety?3 Leptin and ghrelin appear to oppose each other’s actions on appetite, with the most important target being the hypothalamus. Recent studies on obesity suggest the body may become insensitive to leptin. Therefore, animals may continue to feed and further increase their weight, with this loss of satiety.
If you review the three hormones involved in appetite and satiety, it should be obvious that CCK and ghrelin act quickly, with immediate impacts on nutrient and energy balance. By contrast, leptin has a longer lasting impact on nutrient and energy balance.
12.4 Absorptive and Post-Absorptive States
Considering most tissues and the nervous system have high energy demands, glucose and carbohydrates appear to be the primary rapid energy supply. It stands to reason that circulating glucose in the blood is highly regulated, and there are many controls and metabolic pathways maintaining circulating levels of glucose over time.
When glucose is used rapidly, the blood glucose levels can drop below normal homeostatic levels and result in hypoglycemia. Common symptoms of hypoglycemia include shaking, sweating, dizziness, mood swings (“hangry”), and loss of consciousness. Notice the neurological problems when you don’t provide enough energy to the brain, again suggesting the importance of glucose to the nervous system. When glucose is supplied beyond the use or storage capacity of the body the result is hyperglycemia. Common problems include the glycation of extracellular proteins and difficulty maintaining osmotic balance.
During digestion (Figure 12.6, below), the body is said to be in an absorptive state. During this time, most nutrients obtained from the digestive system are circulating in the blood and being taken up by cells and tissues. If the meal contains digestible carbohydrates, there are high levels of glucose circulating in the blood during this time. When blood glucose levels are high, the beta cells of the pancreas are stimulated to release insulin. Insulin stimulates uptake of blood glucose into muscle and liver (forming glycogen) and into adipose tissues (forming triglycerides). This process largely ensures hyperglycemia will not occur.
In humans, long-term hyperglycemia is a symptom of Type II diabetes, where the tissues (especially muscle, adipose, and liver) do not respond to normal levels of pancreatic insulin. By contrast, Type I diabetes patients do not produce enough pancreatic insulin to encourage the uptake of glucose by muscle, adipose, and liver.
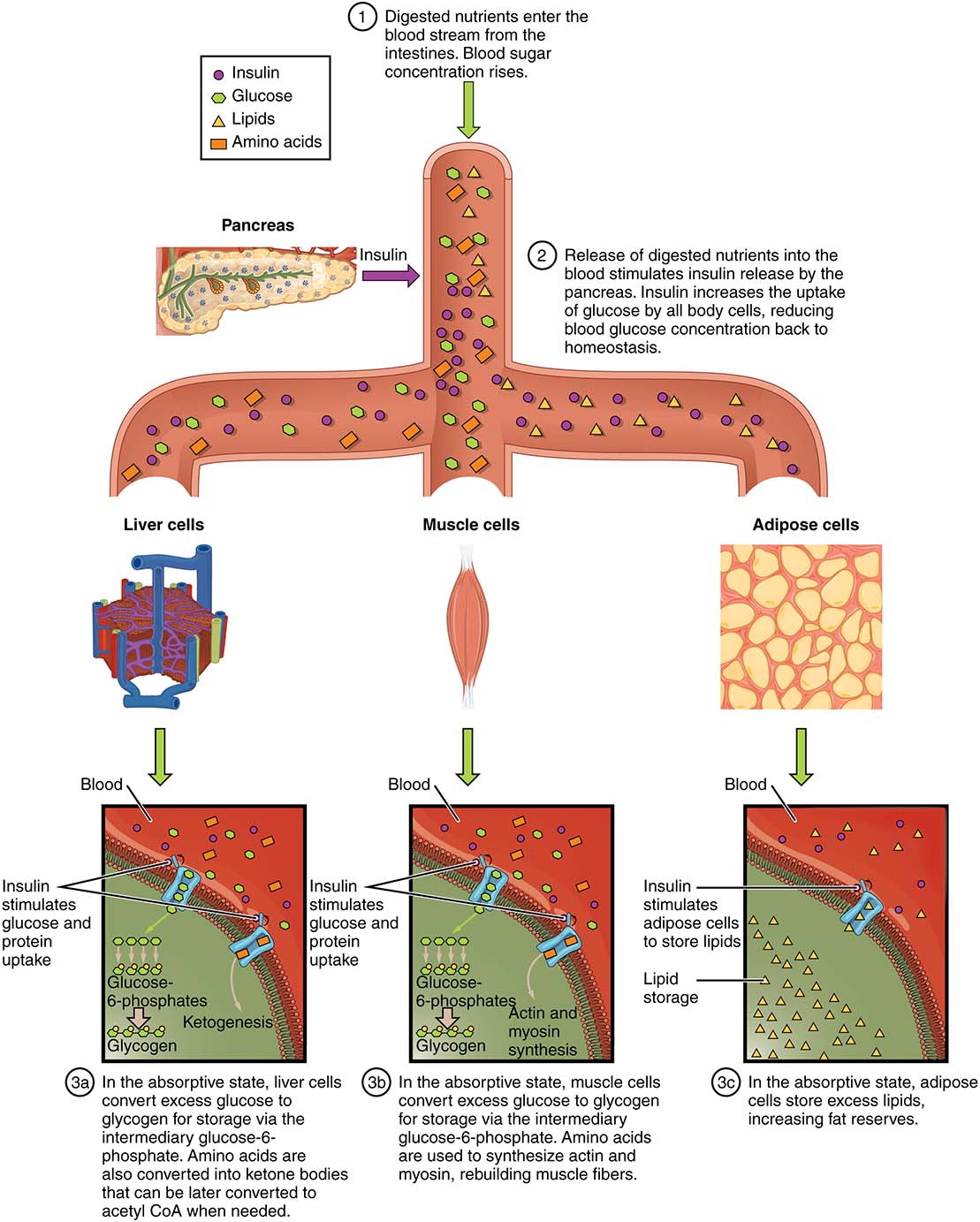
Figure 12.6 Insulin, and the absorptive state. Source: Anatomy and Physiology, Rice University. Download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@11.1. CC BY 4.0, 2018..
A few hours after a meal or following intense exercise, the body enters a post-absorptive state (Figure 12.7, below). During this time, blood glucose levels will begin to decline. Ghrelin may be released to increase appetite or hunger, and the feed-forward response may be initiated in the stomach and intestinal tract (“stomach growling”). If no new food is available and blood glucose levels continue to decline, the pancreas will release glucagon from the alpha cells. Glucagon’s main target is the liver, and it stimulates the liver to break down glycogen, releasing glucose into the blood. Short-term stress, like exercise, will cause a release of epinephrine from the adrenal glands. Epinephrine will further enhance the degradation of glycogen in the muscle (and epinephrine may enhance the liver process). The collective processes of glycogen degradation are known as glycogenolysis and ensure hypoglycemia is avoided.
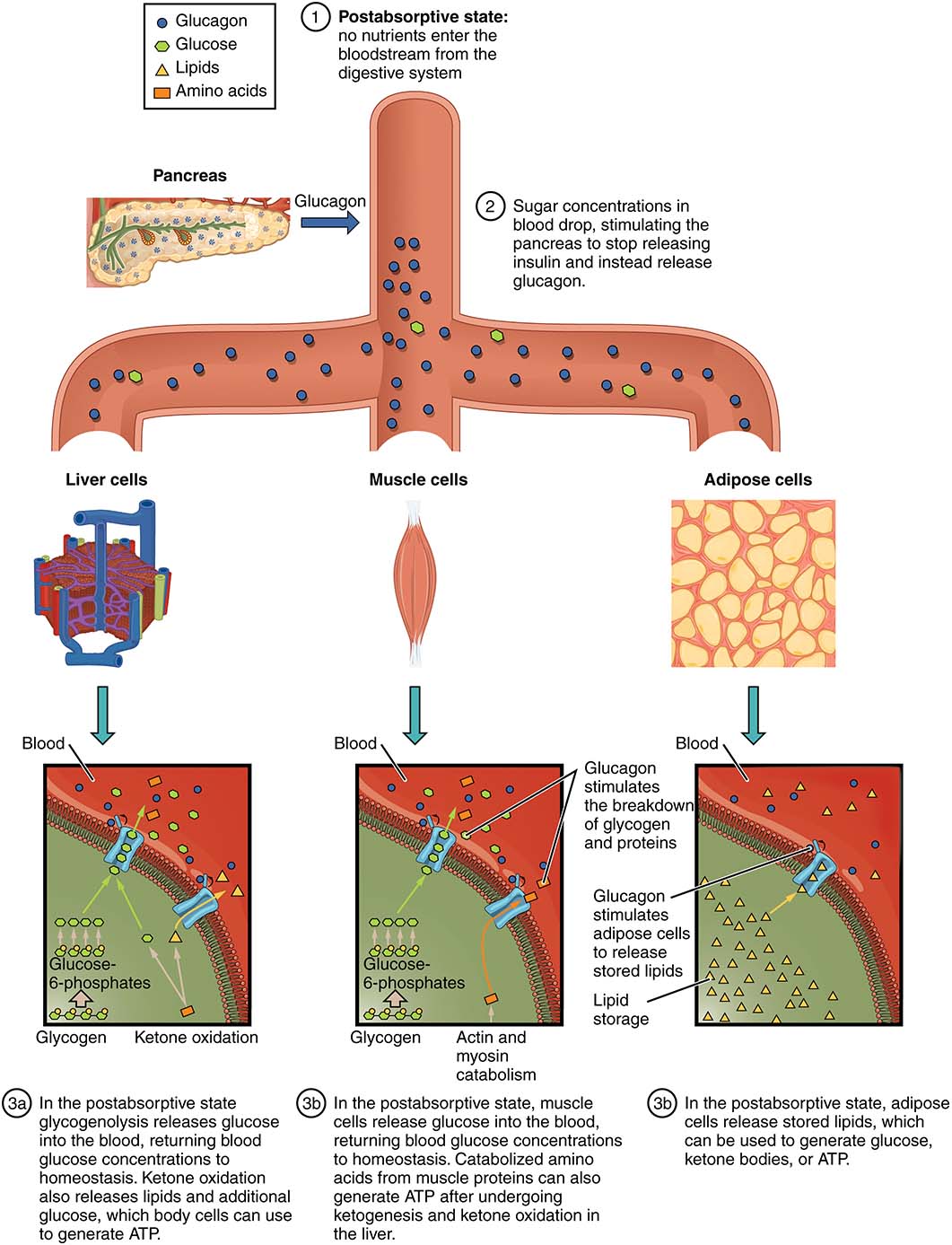
Figure 12.7 Glucagon, and the post-absorptive state. Source: Anatomy and Physiology, Rice University. Download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@11.1. CC BY 4.0, 2018.
If the post-absorptive state continues after the liver has run out of glycogen, the body will make new glucose, preventing blood glucose levels from dropping dangerously low. The metabolic process of making new glucose is called gluconeogenesis (Figure 12.7). During gluconeogenesis, the liver, muscle, adipose and other tissues start creating new glucose by modifying lactate, pyruvate, most amino acids, glycerol, and those fatty acids that have an odd number of carbon atoms. Metabolic feedback mechanisms and allosteric enzymes help to regulate gluconeogenesis. Additionally, long-term stress often is associated with the release of cortisol, or glucocorticoid, from the adrenal glands. High levels of cortisol may initiate gluconeogenesis or enhance the process. If blood glucose climbs, insulin will once again be released to encourage the uptake of glucose from the blood. Therefore, insulin may inhibit gluconeogenesis pathways.
Gluconeogenesis is one of the first signs the body is entering a state of ketosis. If the hunger begins to lead to starvation, or long-term stress continues, the body may begin to target excess protein as an easy source of energy for the nervous system. The danger here is the loss of cellular and tissue function due to the loss of protein. To avoid the depletion of proteins and amino acids in the body, lipid metabolism will increase and go beyond the production of fatty acids or glucose. Lipids will now be converted into ketone bodies (simply known as ketones), supplying the nervous system with an alternative source of fuel. During the state of ketosis, muscle and other tissues can use fatty acids and other organics for fuel, leaving the ketone bodies and any small amounts of glucose for the nervous system, all while avoiding the use of amino acids (as much as possible). The “ketogenic diet” – starving the body of glucose to force the body into ketosis – has become very popular recently, with many individuals swearing by it as a weight loss method with miraculous health benefits. However, the medical studies that have been done so far do not support these claims: There is little evidence of weight loss or health benefits for the vast majority of individuals. The exception is the use of ketogenic diets to control epilepsy in some patients. Where it concerns your health, you should be wary of drastic experiments! Consult a physician prior to making large changes to your diet.
12.5 Metabolic Rate, and Energy Storage
While the exact amounts of energy used are difficult to measure directly, there are many indirect methods available to researchers.
Energy values of foods
It’s possible to get rough estimates of the energy values of food. Knowing how much energy is available from foods is useful in the study of nutrition, diet, metabolism, and ecology. The most common experimental technique burns food in pure oxygen and measures the amount of heat released. For obvious reasons the device is called a “bomb calorimeter”. The standard unit of energy is the Joule, but food energy is more often expressed in kilocalories.
A note on units of energy: One Joule is equivalent to one Newton (kg·m/s2) of force applied to a moving object over a distance of 1 metre, kg·m2/s2 if expressed in kilogrammes, metres, and seconds. One calorie is the amount of energy required to raise the temperature of one gram of liquid water by one degree Celsius. 1 calorie is approximately 4.18 joules4.
Now, here’s the confusing part: The Calorie (uppercase C) listed on food labels is a different unit than the calorie (lowercase C). Nutritionists recognize that a large animal’s demand for energy is quite high, so nutritionists usually convert energy measures into thousands of units. The Calorie (with an uppercase C) is a thousand calories or 1 kcal, while the kilojoule (kJ) is a thousand Joules.
To give an example of the amount of energy in organic nutrients, consider one gram of glucose. Burning one gram of glucose has an energy yield of approximately 4 kcal/g, or roughly 17 kJ/g. If the average human requires about 2000 C/day (or 2000 kcal/day) this person would have to consume 500 grams of glucose (or drink 15 cans of cola) to meet basic metabolic demands.
Table 12.1 Energy yields of major nutrients
|
nutrient |
energy released when combined with O2 |
|
carbohydrate |
4 kcal/g carbohydrate |
|
fat |
9 kcal/g fat |
|
protein |
4 kcal/g protein |
Remember that the products of aerobic respiration are water (H2O) and carbon dioxide (CO2). If you take a look at the approximate chemical formula for each of these nutrients, you’ll notice that the amount of energy released per unit of oxygen is somewhat similar for carbohydrates as for fats. The energy yield for burning protein in the body is a bit less because there’s a substantial energy cost to detoxifying and excreting the waste nitrogen.
The energy values printed on food labels are usually calculated from known values like those in Table 12.1, instead of being measured experimentally for every food.
Metabolic rate
The basic metabolic energy demand of an animal is called the basal metabolic rate (BMR). Measuring BMR, called calorimetry, can be performed directly or indirectly depending on the experimental design. Direct calorimetry typically measures the amount of heat loss by the animal’s body over time and relates this to the body mass of the animal and to the amount of food consumed over time. Indirect calorimetry can be performed in many ways, but one common methodology is to measure the amount of oxygen consumed by an animal over a certain amount of time. Since most metabolic pathways are aerobic, measuring oxygen consumptions give researchers a good estimate of metabolic rate. Again, this may be related to the mass of the animal or used to estimate the amount of food required to maintain metabolism.
A major influence on basal metabolic rate is the thyroid gland and its two hormones, triiodothyronine (T3) and thyroxine (T4). Thyroid hormones influence all tissues in the body by influencing mitochondrial activity and increasing oxygen demands. These hormones also have impacts on glycogen storage and degradation, and growth and development. The production and regulation of these hormones is control via the endocrine axis and feedback mechanisms of the thyroid on the endocrine axis (Figure 12.8, next page). Additionally, thyroid hormone production is heavily influenced by nutrition and energy levels in the body. For example, if body temperature drops, this will influence the hypothalamus to release the neurohormone, thyrotropin releasing hormone (TRH). This stimulates the anterior pituitary to release thyrotropin, or thyroid stimulating hormone (TSH). TSH targets the thyroid gland, stimulates the production of the T3 and T4, through the uptake of iodine and modification of the amino acid tyrosine. Can you guess how many iodine molecules are incorporated into T3 and T4? As mentioned previously, T3 and T4 will increase BMR and generate heat within the body.
If excess thyroid hormones are generated, negative feedback on the anterior pituitary and hypothalamus will slow the production of TRH and TSH, thereby lowering thyroid hormone production. A dietary supply of iodine is essential to produce thyroid hormones, and in the absence of iodine, a positive feedback loop within this pathway occurs. This will lead to an over stimulation of the thyroid gland and the incorporation of tyrosine will continue, without the production of thyroid hormones. This leads to a swelling of the thyroid gland known as goitre. Next time you are at the grocery store to pick up a box of salt, notice what has been added to the salt?
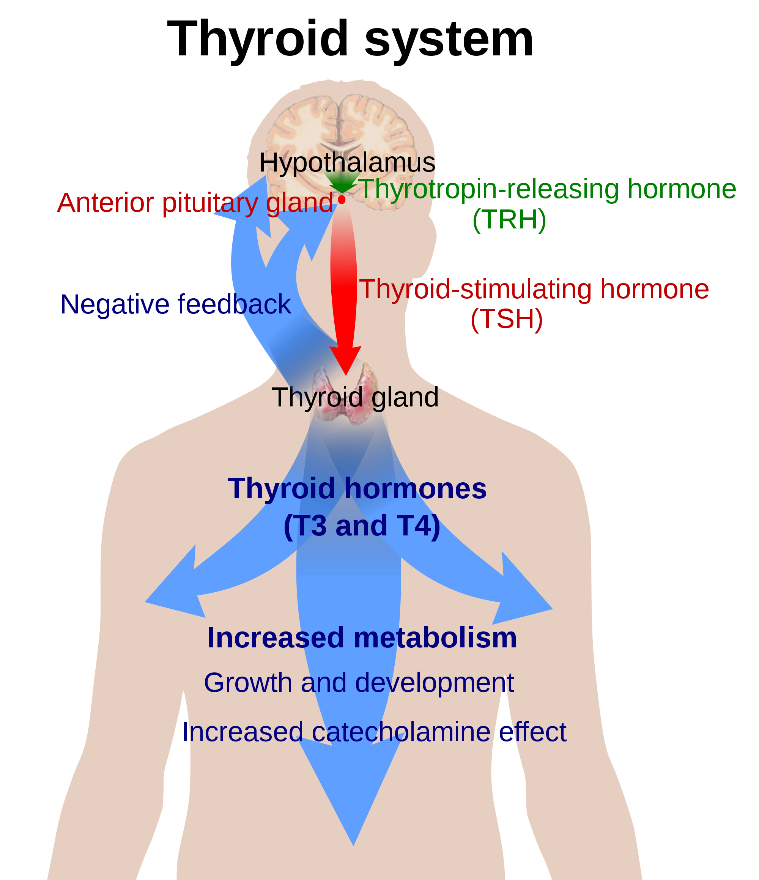
Figure 12.8 Endocrine regulation of the thyroid gland. Source: Mikael Häggström, https://commons.wikimedia.org/wiki/File:Thyroid_system.svg CC0 public domain, 2009.
Based on calorimetry studies many other factors control the metabolic rate of an animal, besides the thyroid. For example, good experimental design for a calorimetry study will weigh the animal, ensure the animal is at rest or inactive, ensure the animal is kept within a standardized ambient temperature (for example, 22 °C for humans), and ensure the animal is in a post-absorptive state. From this short list, can you think of a few factors changing your metabolic rate over the course of a day?
By studying all the animal systems, have you noticed a trend? Larger animals have larger hearts, larger lungs, larger brains, larger muscles, larger everything! It should not be surprising that the BMR of a larger animal increases with increased tissues. To support this concept, indirect calorimetry using oxygen consumption will clearly result in a direct relationship between an increase in oxygen consumption as the body mass of the animal increases. However, this is not a complete picture of metabolism. When researchers measure the oxygen consumption per gram of animal tissue, an indirect relationship appears. Smaller animals, per gram of tissue, consume more oxygen suggesting smaller animals have a higher BMR than larger animals. One obvious hypothesis is the tissues of smaller animals rely more on aerobic respiration, while more tissues in larger animals are more anaerobic. However, the exact reasoning for this indirect relationship is unclear, and currently the topic of much research.
What is clear from calorimetry studies? BMR and body weight are directly related to tissue activity, especially that of the nervous system and muscle. So, the higher the brain function and the more physically active the animal is, the higher the metabolic rate, the more nutrients consumed, and the less nutrients stored during post-absorption. If animal becomes inactive, metabolic demands decrease, and more of the absorbed nutrients will end up being stored. Voluntary activities such as studying for an exam (yes, that is voluntary, too voluntary for some!) and then taking a break to play a sport will dramatically increase your metabolic rate. By contrast sitting on a couch and watching TV or falling asleep with textbook in hand will dramatically lower metabolism (contrary to popular believe you don’t learn via diffusion or osmosis from the textbook).
From this aspect body mass or weight seems simple, almost like a bank account. If you put more money in your account than you spend, your bank account grows. If you spend more money than you save, your bank account shrinks. While body weight seems simple, the exact regulation over weight is highly complex and not well understood. We previously discussed leptin and its impacts on appetite, and the thyroid hormones and their impacts on overall metabolism. However, researchers must acknowledge the psychological effects of food, which can completely override the physiology of the body. As an example, sit and think of your favourite food. Concentrate on it: Recall the smell of it, the taste of it, and the feeling you get while eating it. Is your stomach growling yet?? Even though you may not require nutrition, these “comfort” foods have huge influences on our diet and body mass regulation. Other behaviours like hyperactivity or ADHD (attention deficit hyperactivity disorder) can be associated with weight loss and lack of appetite even when the body is deficient in nutrients.
Depending on the animal, various states of activity will occur throughout the life. Younger animals are generally more active and have higher BMR than older animals. Some animals, like shrews, hummingbirds, and high-performance athletes, are highly active and have extremely high BMR and extremely high dietary requirements. These organisms must source food continually to support their bodies. Other cold-adapted animals, like bears, ground squirrels, and northern tree frogs, often become highly inactive during period of cold or periods when food is not readily available. There are many terms for the lowered states of activity, including hibernation, torpor and estivation. Hibernation is the reduction of metabolism well below normal levels. The exact amount of reduction can vary from animal to animal. Bears may exhibit a small reduction, where as ground squirrels and frog may reduce their metabolic output to near zero. Torpor is a reduction in metabolism over a short duration, from a few hours to a few days. Hummingbirds may perform overnight torpor when many flowers are closed or when nectar is in short supply. Estivation is a reduction in metabolism in response to heat. Desert animals will exhibit periods of estivation during the hottest part of the year.
12.6 Control of Body Temperature
Besides the production of ATP, another major energy by-product of metabolism is heat or an increase in body temperature. Temperature is a measure of available heat, or heat and energy displacement. If your body is cooler than the surrounding environment your body gains heat or energy. If your body is warmer than the surrounding environment, your body loses heat. Outside of metabolism, there are four main physical factors controlling the movement of heat to and from an animal’s body: conduction, radiation, convection, and evaporation. Conduction is heat gained or lost through direct contact. Heated molecules have more energy than cooler molecules, as high-energy molecules contact lower energy molecules there is a transfer of energy from the higher to the lower energy molecule. If you put your hand on the exterior of a university building, in the middle of winter, you will have conducted much heat from your hand into the building. Radiation is the gain or loss of energy via electromagnetic emissions. The sun does not directly touch you, yet its emitted energy can warm your body. Convection occurs when a fluid (gas or liquid) gains heat and changes density. Warmer fluids being less dense than cooler fluids, tend to rise or float. As these warmer fluids rise, they will leave space for fluids to replace them and they will displace other fluids as they rise. Generally, it is the fluids from above, having lost their energy and having increased their density that fall to replace the rising and expanding fluids. As long as there are no major disturbances to this fluid system, currents of flow begin developing, or convection currents are established. Convection currents, although difficult to see, will be established on the surface of your body. To observe this more clearly, watch a pan of soup heat up and observing the rise and fall of materials within the soup.
Animals that are large enough and have high enough levels of metabolic activity often produce enough heat to make the temperature deep inside their body significantly warmer than their environment. Animals that use metabolic activity to maintain a body temperature significantly warmer than their environment are called endotherms.
Do all animals strictly obey the categories created by biologists? What do you think – of course not! There are a lot of animals that don’t fit into tidy categories of “endotherm” and “ectotherm”, being able to use their metabolism to raise their body temperature part of the time, and allow it to drop at other times, or warm just part of their body. Examples include some species of fish, bees, bats, hummingbirds, and sea turtles.
Thermoregulation is the homeostatic regulation of body temperature. Some animals highly regulate their body temperature through metabolic output, but others do not. Those animals that maintain a high BMR and use this to generate most of their own heat are term endotherms. If an animal constantly relies on outside sources of heat to supplement their metabolic output, they are termed ectotherms. Ectotherms animals can be further divided into two sub-categories. Ectothermic poikilotherms (the most common in conversation) not only rely on outside sources of heat but also vary their body temperature and metabolism in direct response available heat energy in the environment. The common tree frog will become more active in warmer environments and less active in cooler environment, as will most insects, molluscs, fish and reptiles. Ectothermic regulators, or ectothermic homeotherms, are animals that keep the body temperature within a very narrow range because their environment is consistently the same temperature. Most deep-sea sharks are also considered ectothermic homeotherms, as they are adapted to the constant cold water of the deep sea and do not function well if removed from that environment.
As discussed in the previous section, voluntary activity will increase metabolism, thereby, increase the body temperature of both ectothermic and endothermic animals. Active muscle is one of the largest contributors to heat generation in the body. If you have exercised recently, you will have noticed the direct relationship between an increase in skeletal muscle activity and an increase body temperature. Muscle activity can be increased through involuntary actions when the body requires more heat. Shivering, or shivering thermogenesis, is involuntary muscle activity in response to a lower body temperature and is especially prevalent in endotherms. Thermoreceptors in the skin and in the core will detect small changes in temperature. The lower temperature stimuli are relayed to the hypothalamus. The hypothalamus will eventually trigger motor responses within the skeletal muscle, causing the muscle to twitch at relatively high frequency. As with exercise, the more active skeletal muscle generates a tremendous amount of heat for the body. See Figure 12.9, next page.
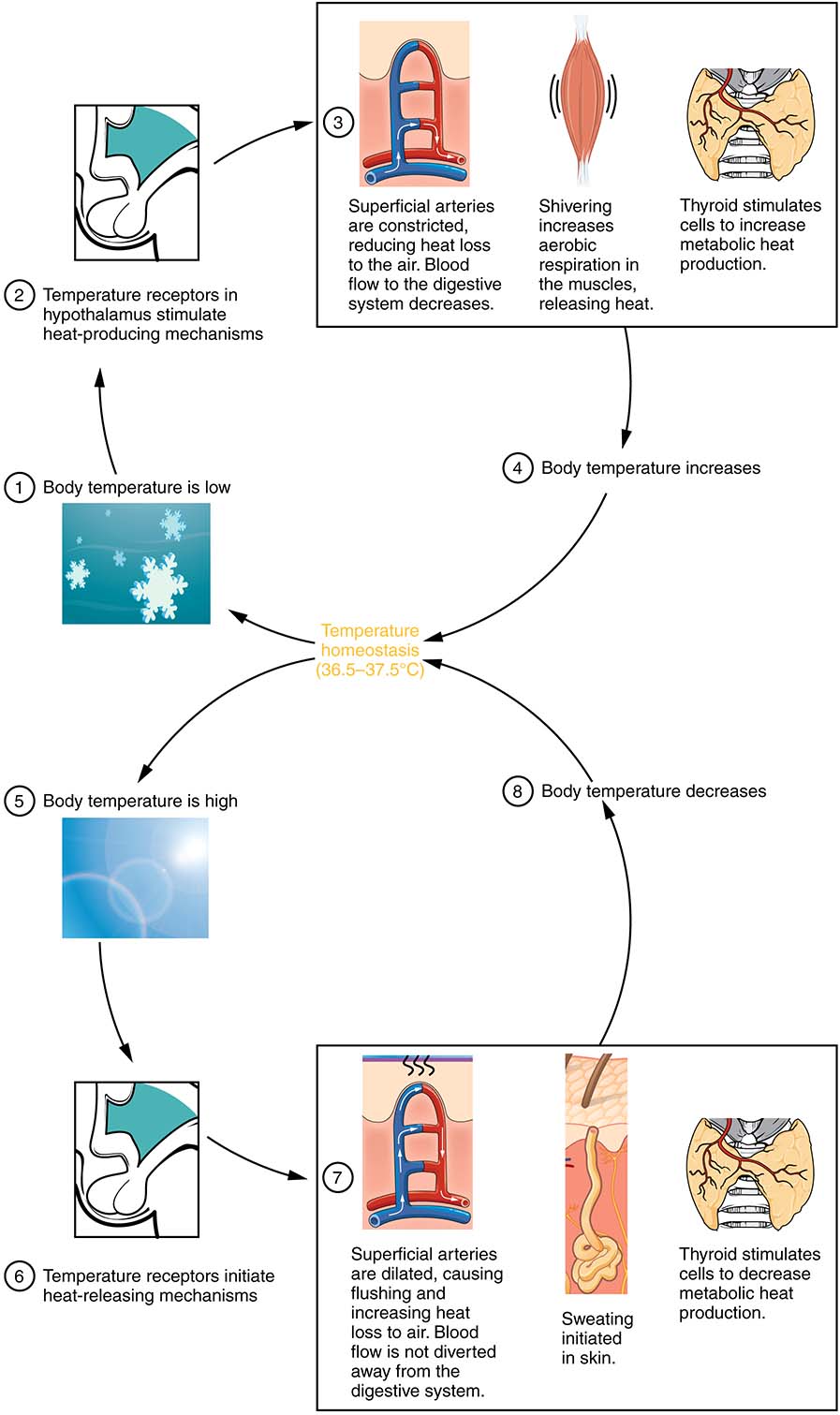
Figure 12.9 The hypothalamus, metabolism, and body temperature. Source: Anatomy and Physiology, Rice University. Download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@11.1. CC BY 4.0, 2018.
Other regions of the body can also be used to generate heat for an animal. An active digestive system, including the pancreas and liver, can generate heat for the body, during a process known as food-induced thermogenesis. Additionally, some animals (especially young endotherms) have a type of highly vascularized adipose tissue known as brown fat. The cells of this tissue also have excessive numbers of mitochondria. If the body temperature drops, the hypothalamus uses the sympathetic division of the PNS and the release of norepinephrine to activate brown fat. Once metabolically active, brown fat will generate heat for the body, in a process known as non-shivering thermogenesis. The cellular mechanisms behind non-shivering thermogenesis seems to rely on altering oxidative phosphorylation pathways within the brown fat mitochondria, especially decoupling ATP-synthase from its source of energy, the H+ ion gradient. It appears an alternate protein channel for the flow of H+ ions, called thermogenin, can be placed along the inner membrane of the mitochondria. This allows the flow of H+ ions back into the mitochondrial matrix without activating ATP-synthase or generating ATP. Instead, the flow of H+ ions contributes solely to heat production.
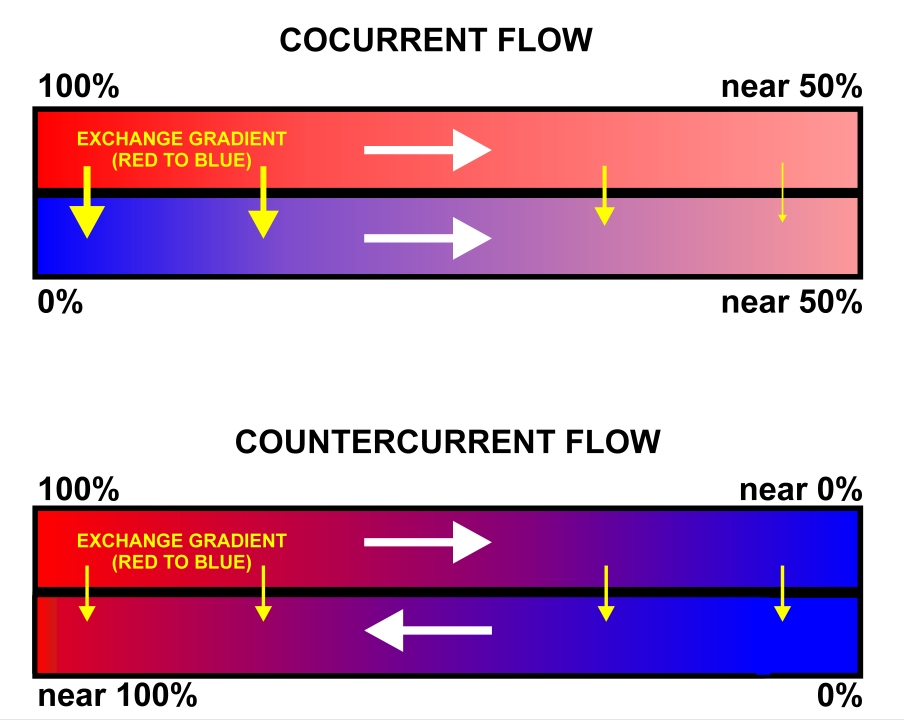
Many other mechanisms are involved in heat production or the retention of heat. Developing sub-cutaneous adipose tissue will act as an insulating layer, slowing heat loss due to radiation and conduction. This is obvious in arctic mammals with their layers of blubber, but subcutaneous adipose tissue is present in most vertebrates. Insulation may be further increased through the development of thick layers of fur or feathers, in mammals and birds. These materials will create boundary layers next to the skin, especially reducing heat loss due to convection. You will recall that peripheral blood vessels may experience vasoconstriction to reduce blood flow and heat loss from the skin, see Figure 12.9. Further modifications to the circulatory system include networks where the artery delivering blood to the periphery runs beside the vein returning blood. This allows counter current heat exchange to occur, where the warm arterial blood reheats the cool venous blood as it returns to the body core from the extremities (Figure 12.10). Finally, continual heat loss will lead to many problems, including reduced enzyme function and ice nucleation or freezing of bodily fluids. Enzymes function will be discussed later, but the prevention of ice-nucleation is critical to preventing damage to cells and tissue. Many animals will introduce high levels of salts or sugars into the tissues to act as antifreezes. Other animals produce unique molecules that severely limit ice nucleation in tissues.
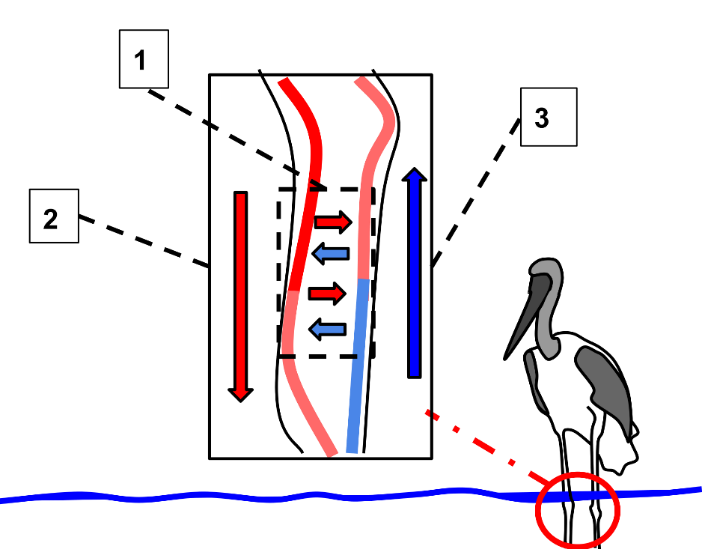
An animal experiencing high temperature will try to reverse or avoid many of the previous adaptations. Loss of subcutaneous adipose tissue increases heat radiation, and loss of heat through conduction. Reduced density of hair or feathers increases heat loss due to convection. Other animals produce heat-shock proteins, preventing enzymes and other proteins from denaturing and reducing metabolism to dangerous levels.
Yet, when it involves cooling, there is one mechanism use by most animals which incorporates all physical heat exchange mechanisms, this is the use of water and concept of evaporation. Most animals will naturally look for a source of water when their bodies become over-heated. Water has a high specific heat and a high heat of vaporization. When an animal enters a body of water, much heat is lost due to radiation, conduction and convection interactions between the animal’s body and the water molecules. Liquid water has a high specific heat (the heat energy required to raise one unit mass of a substance by one degree Celsius, this is approximately 1 cal/g·°C for water), therefore it can absorb much heat from an animal’s body. However, when the animal leaves the water the concept of evaporation has an equally important influence on thermoregulation. To cause water to evaporate from the skin of an animal, a tremendous amount of heat (heat of vaporization) needs to be added to liquid water to create gaseous water. Since most of the heat comes from the body of the animal, the net effect is a reduction in the animal’s body temperature. This process, known as evaporative cooling, can be increased by increasing blood flow to the periphery through vasodilation or by adding more water to the surface of the skin or to other surfaces with direct exposure to the environment. Some animals will sacrifice saliva and lick their skin to increase evaporative cooling, while others will increase respiration rates by breathing heavily through the mouth, known as panting in mammals or gular fluttering in birds. Some animals, including humans, can secrete enough water from sweat glands to achieve significant cooling. During sweating, thermoreceptors detect an increase in body temperature. This information is relayed to the hypothalamus and stimulation of the eccrine glands, as well as, vasodilation occurs. Water in the form of sweat increases on the skin’s surface as does the amount of heat delivered to the skin, ensuring effective evaporative cooling occurs (Figure 12.9).
Finally, most metabolic pathways and heat production are affected by enzymes, and temperature can greatly affect enzyme function. Enzymes’ three-dimensional nature typically suggests they will only function in certain environments and will denature (or lose their 3D functional shape) outside those specific environments. Recall the enzyme pepsin. Pepsin is active in the low pH environment of the stomach but is denatured once it encounters the neutral pH environment of the small intestine. Similarly, enzymes often have optimal functional temperatures (for humans most enzymes function best between 36 °C and 37°C) and denature outside these optimal temperatures. Does this partially explain why health practitioners are so concerned about prolonged fevers or severe fevers when you are ill? However, some interesting studies on fish metabolism have demonstrated the amazing capacity of animals to adapt or acclimatize to different environmental influences. Researchers expected most fish, being ectotherms, to slow their metabolic activity during winter or in cold water due to a loss of enzyme function, and increase their metabolic rate in summer, or warm water as enzymes began to work optimally again. They measured activity of fish at different water temperatures, predicting fish in cold water to be less active than fish in warm water (review the poikilotherm discussion). However, what most researchers have found is the same fish are quite active in both cold water and warm water. What appears to be happening is a shift in enzyme structure and function through gene regulation. Prolonged exposure to cold will result in the production of cold tolerant enzymes, while prolonged exposure to warmer water will shift the body toward heat tolerant enzymes. Life is truly amazing!
12.7 Summary
- Energy in the body obtained through a series of metabolism reactions, including cellular respiration.
- Metabolic reactions can build materials or degrade material, use energy or donate energy.
- Cellular respiration occurs in and around mitochondria and can break down all nutrients to release energy, with the typical order of use being carbohydrates, lipids, then proteins.
- Intake of food is often controlled by appetite or satiety centers in the hypothalamus, which is regulated by many signalling molecules including CCK, ghrelin and leptin.
- Digested food must be delivered by the circulatory system to cells and tissues, and should be in simple forms like glucose, fatty acids and amino acids to be metabolized.
- Initial deliver and uptake of nutrients into the tissues is called the absorptive state, here insulin plays a large role.
- Continued metabolism of nutrients occurs in the post-absorptive state, here glucagon, epinephrine and cortisol play significant roles.
- Metabolism is often measures as basal metabolic rate (BMR), or the amount of energy used over time.
- Direct or indirect calorimetry can be used to establish BMR.
- The hypothalamus also controls the thyroid gland, which has direct influence on BMR through the production and function of T3 and T4 hormones.
- Weight gain or loss is directly influence by metabolism but is difficult to regulate due to psychosocial and behavioural patterns.
- The hypothalamus has controls over tissues activity which directly influence BMR and heat production in an animal’s body, including the effects of shivering, food induced thermogenesis and non-shivering thermogenesis.
- Endotherms are animals which have greater internal controls over metabolism and heat production, where as ectotherms are more reliant on the environment.
- Many physical factors can influence the temperature of an animal, including radiation, conduction, convection and evaporation.
- Water and evaporative cooling play important roles in animal body temperature.
- Enzymes control metabolism, yet enzymes themselves can be regulated by metabolism and heat production due to the 3D nature of enzymes and the denaturing of enzymes.
12.8 Glossary
Absorptive state – period following digestion when new nutrients are circulating in the blood and being incorporated into cells and tissues to be used in metabolism
Adenosine triphosphate or ATP – nucleotide used to phosphorylate other molecules thereby energizing other molecules, sometimes called the energy currency of cell
Aerobic respiration – metabolic reactions delivering high-energy outputs in the presence of oxygen, typically requiring the presence of mitochondria
Anabolic – chemical reactions building larger molecules from simpler molecules
Anaerobic respiration – metabolic reactions occurring in the absence of oxygen, often delivering lower energy outputs and not requiring mitochondria
Appetite – the feeling of hunger or the desire to consume food
Basal metabolic rate or BMR – the minimum amount of energy the body requires to maintain metabolism while at rest
Brown fat – a mitochondria-rich tissue that is specialised for heat production, most abundant in infant mammals
Calorie – a unit of energy measure: lower case calorie is defined as the amount of energy required to raise one gram of water by one degree Celsius, upper case Calorie is the nutritional definition and is thousands of calories
Calorimetry – the process of studying and measuring the amount of energy of chemical reactions or energy used by a body
Catabolic – chemical reactions degrading larger molecules into simpler molecules, often releasing energy in the process
Cholecystokinin or CCK – a small peptide signalling molecule produced by the duodenum targeting the liver and pancreas to stimulate lipid and protein digestion, and targeting the hypothalamus to induce satiety
Conduction – in terms of energy the movement of energy from one material to another through contact, high energy molecules transferring energy to lower energy molecules through collision
Convection – the transfer of energy due to the movement of liquids, as liquids change positions due to changes in density
Cortisol or glucocorticoid – a steroid hormone produced in the cortical region of the adrenal gland is response to low levels of glucose in the blood, and responsible for stimulating gluconeogenesis
Counter current exchange – an exchange system where fluid on either side of a thin barrier flows in opposite directions, resulting in highly efficient transfer of either heat or a substance from one fluid to the other
Eccrine glands – a common sweat gland used in evaporative cooling
Ectotherm – an animal relying on environmental heat to help maintain metabolism
Endergonic – a non-spontaneous reaction or a reaction requiring large amounts of energy to form products
Endotherm – an animal generating most heat internally to help maintain metabolism
Evaporative cooling – the process of heat loss from the body due to conversion of liquid water to gaseous water on the surface of a body
Exergonic – a spontaneous reaction or a reaction releasing large amounts of energy during the formation of products
Food-induced thermogenesis – heat generated within the body due to an increase in metabolism during the digestion and absorption of food
Ghrelin – a small peptide signalling molecule produced by the stomach and small intestine which can excite the appetite centers in the hypothalamus to stimulate the feeling of hunger
Gluconeogenesis – conversion of non-carbohydrate organic compounds into glucose
Glycogen – the main carbohydrate storage molecule in the liver and muscle, also called animal starch
Glycogenolysis – the degradation of glycogen to increase glucose levels in the blood
Goitre – excessive swelling of the thyroid gland due to the lack of iodine in the diet
Heat-shock proteins – chaperone proteins helping other proteins and enzymes fold properly during times of stress
Ketone bodies or ketones – products of lipid digestion during times of prolonged stress or during periods of starvation, acting as an alternative source of fuel for the nervous system and other tissues
Hyperglycemia – having above normal levels of glucose circulating in the body
Hypoglycemia – having below normal levels of glucose circulating in the body
Joule – a unit of energy measure, often represented in units as kilograms multiplied by metre squared divided by seconds squared
Ketosis – a state of the body where metabolism switches from using carbohydrates as the main fuel to ketone bodies, often occurring during periods of prolonged stress or starvation
Leptin – a small peptide signalling molecule produced by adipose tissue, often opposing ghrelin and leading to a satiety response
Non-shivering thermogenesis – increased metabolic output in brown fat and other tissues to increase heat generation in the body without muscle contraction
Oxidative or oxidation – chemical reactions where electrons are donated to other molecules, paired with reductive reactions
Poikilotherms – ectothermic animals which vary their body temperature and metabolism as the ambient temperature changes
Post-absorptive state – a state of the body when the digestive system is empty and stored nutrients are being metabolized and released into the blood
Radiation – the transfer of energy through electromagnetic emissions
Reduction or reductive reactions – chemical reactions where electrons are gained from other molecules, paired with oxidative reactions
Satiety – the feeling of being full, or the lack of hunger
Shivering thermogenesis – high frequency twitching of muscle to generate heat for a body, especially prevalent in endotherms
Sub-cutaneous adipose tissue – a layer of fat found just under the skin acting as a layer of insulation for the body
Thermogenin – a hydrogen ion channel found in the mitochondria of brown fat cells allowing hydrogen ions to bypass ATP-synthase and generate heat, rather than generating ATP
Thermoregulation – the homeostatic regulation of body temperature
Thyroid gland – one of the classical human endocrine glands found in the throat region of humans, responsible for production of thyroxine (T4) and triiodothyronine (T3) and important in regulating metabolic output of the body
Thyroid stimulating hormone or thyrotropin – a signalling molecule, released from the anterior pituitary, stimulating the thyroid gland
Thyrotropin releasing hormone – a signalling molecule, released from the hypothalamus, stimulating the anterior pituitary to generate thyrotropin
Thyroxine or T4 – one of two thyroid hormones regulating metabolism, based on the amino acid tyrosine and containing four iodine molecules
Triiodothyronine or T3 – one of two thyroid hormones regulating metabolism, based on the amino acid tyrosine and containing three iodine molecules

