5. Signal Transmission and Networks in the Nervous System
Chapter 5 – Signalling and Networks in the Nervous System
- 5.1 Introduction
- 5.2 Electrical Signalling in Neurons
- 5.3 Signal Integration in a Neuron
- 5.4 Circuits
- 5.5 Reflexes
- 5.6 Glossary
5.1 Introduction
Seals know it. Pilot whales know it. Puffins know it. Even sharks know it: Calamari – squid – are delicious. So, what can you do if you’re small, delicious, and exposed in the open ocean? Lightning-fast reflexes are your key to survival.
Jet propulsion allows squid to travel at 40 km/h, as fast as a shark, and squid sometimes even become airborne above the surface of the water, like flying fish. Squid have large, complex eyes with which to see an approaching predator, but this information needs to get to the muscular mantle as quickly as possible – it’s the mantle that contracts to create a jet of water. Specialised motor neurons in squid have evolved a way to increase the speed of electrical signals – their axons are many, many times wider in diameter than a normal axon.
The squid giant axon that controls part of the squid’s water propulsion system enabled the pioneering studies on signalling in neurons. The squid giant axon has a diameter sufficiently large – up to 1 mm in diameter, typically around 0.5 mm – to permit the introduction of electrodes for measuring electrical changes across the cell membrane. Electrical changes are detected with a voltmeter to record the difference in electrical potential (voltage) between the microelectrodes located inside and outside the axon (The concept of electrical potential is explained on the next page). Membrane potentials are measured in millivolts (mV) as a function of time, in milliseconds (ms).


Voltage is the amount of work required to move a charged particle against an electrical field, or equivalently, the amount of work that can be done by charged particle when it moves from point A to point B an electrical field. Think of the work you do to pull a toboggan up a hill, and the work that the gravitational field does on you and the toboggan as you ride it down the hill.
Charged particles themselves create the electric field, such that like charges repel and opposite charges attract. The voltage across a cell membrane is called the membrane potential. The unit of voltage is the volt (V), in honour of the Italian physicist Alessandro Volta (1745–1827) who invented the voltaic pile, now known as a battery.
Electrical resistance is a measure of the difficulty to pass an electric current through an electrical conductor. Substances that have extremely high resisances are called insulators. The unit of electrical resistance is the ohm (Ω), named after German physicist Georg Simon Ohm.
Electrical conductance is the ease with which an electric current passes through an electrical conductor. The unit of electrical conductance is the siemens (S), named after the German inventor Ernest Werner von Siemens.
Capacitance is the ability of a body to store an electrical charge. The unit of capacitance is the farad (F), named after the English physicist Michael Faraday. Because opposite charges attract each other, two conductors separated by a thin insulator will have a high capacitance. Where have you seen such a setup? The cell membrane, of course!
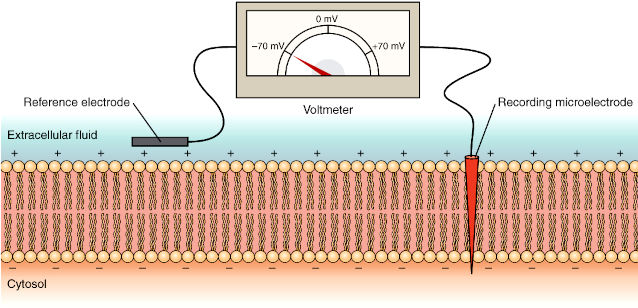
5.2 Electrical Signalling in Neurons
Electrical changes are transmitted from neuron to neuron or from neurons to other non-neural cells, such muscle or gland cells. In order to understand how electrical signals are transmitted in an individual cell, you need to become familiar with the function of ion channels in the plasma membrane. They are embedded in the membrane and allow passive movement of ions in and out of the cell. They include both voltage-gated channels and ligand-gated channels, which were introduced in Chapter 3. There are many different kinds of voltage-gated channels and ligand-gated channels, as well as leak channels that stay open, and yet other kinds of ion channels that open and close in response to intracellular signals.
5.2.1 Resting Potential in the Neuron
Cells are enclosed by a lipid bilayer, which is a strong electrical insulator, but have embedded ion channels and pumps that make the cell electrically active. The electrical currents relevant to nervous system signalling are carried by ions. Neurons are electrically polarized, meaning that there is a difference in charge between inside and outside of the cells. Like a battery, there are positive and negative poles. These poles are located outside and inside of the plasma membrane which acts as a barrier separating charges. At rest, the interior of a neuron is more negative than the exterior. This difference, the resting membrane potential, is around -70 mV.
-70 mV might not sound like much, but you have to remember the small size scale of biological membranes. It actually corresponds to an intense electrical field, the same intensity that would cause sparking in air. However, the cell membrane is tough, so it takes 200 mV to rupture a biological membrane.
The differences in charge are established by an uneven distribution of anions and cations between the two sides of the cell membrane. The main ions that establish this distribution are Na+, K+ and Cl–, and the negatively charged intracellular proteins and RNA trapped inside the cytoplasm (Table 5.1). Because of their size, proteins and other macromolecules do not readily move through the plasma membrane.
| Ion | Extracellular | Intracellular |
|---|---|---|
| Na+ | 145 | 15 |
| K+ | 5 | 150 |
| Cl– | 100 | 7 |
| Anions in macromolecules | <1 | 65 |
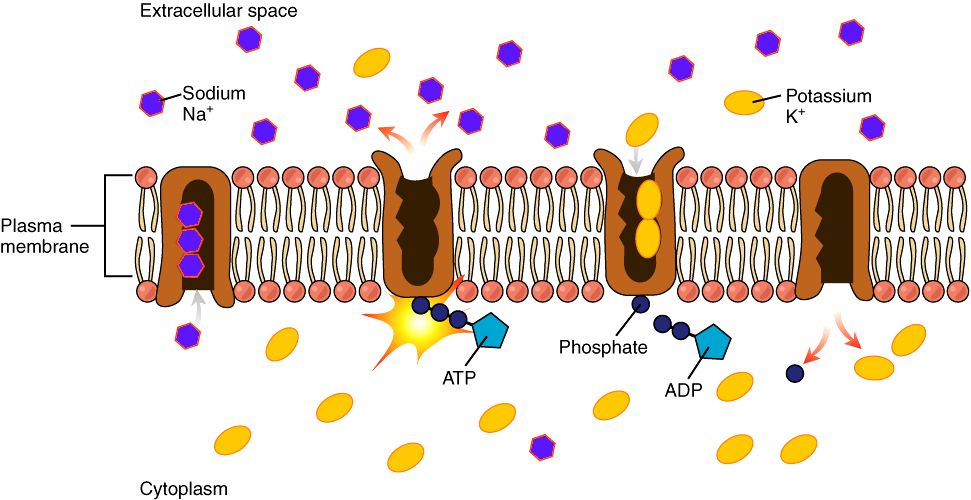
Two important components of the plasma membrane contribute to establish the resting membrane potential:
- The Na+/K+-ATPase – The Na+/K+-ATPase can move 3 Na+ out of the cell for every 2 K+ moved into the cell. This ion pump uses ATP hydrolysis to provide the energy to pump both ions against their concentration gradients. In this way, the Na+/K+-ATPase pump establishes gradients for Na+ and K+. But the Na+/K+-ATPase it makes only a small direct contribution to the charge difference across the plasma membrane in healthy cells. Its main contribution to the resting potential is by storing energy in the form of a K+ concentration gradient.
- The potassium leak channels – K+ leak channels embedded in the membrane allow passive movement of K+ in and out of the cell. Leakage of other ions is relatively small. Thus, at rest the membrane is more permeable to potassium than to sodium. Think for a moment what this means for the resting membrane potential.
The Na+/K+-ATPase is constantly pumping Na+ out of the cell and K+ into the cell. Therefore, the concentration of Na+ is higher outside the cell than inside, and the K+ concentration is higher inside of the cell than outside. Because there are more K+ ions inside than outside, the ion diffuses out of the cell through the open channels down its concentration gradient, leaving behind an unbalanced negative charge that tends to pull K+ back into the cell. This imbalance creates two forces:
- The concentration gradient, and
- An overall electrical gradient.
Both forces constitute the electrochemical gradient. Potassium ions diffuse through K+ channels until the concentration gradient pushing K+ out of the cells exactly equals the electrical charge pulling K+ back into the cell. At this equilibrium point the electrochemical gradient is zero, and there is no net movement of K+. Thus, the membrane potential at this point is the K+ equilibrium potential of -70 mV.
Overall, K+ is the only ion that is free to move when the neuron’s cell membrane is at rest. Therefore, potassium will drive the cell’s membrane potential toward the potassium equilibrium potential. Thus, K+ leak channels are largely responsible for the membrane’s resting potential.
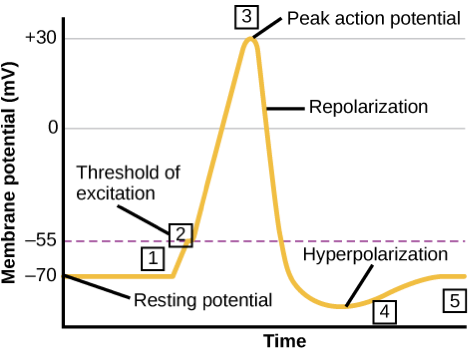
5.2.2 Action Potentials in the Neuron
In the activated neuron, the signal is generated in the form of action potentials, which are short-lasting changes of electrical voltage across the cell membrane. The membrane potential during and action potential rapidly rises and falls, following a consistent trajectory. Generation of action membrane potentials is a property exclusive to a few types of animal cells, called excitable cells, which include neurons, muscle cells, and endocrine cells.
- In neurons action potentials are also known as “nerve impulses” or “spikes”. A neuron that emits an action potential is often said to “fire”;
- Action potentials play a central role in neural cell-to-cell communication, as well as in communication between neural and other cells of the body;
- In muscle cells, an action potential is the first step in the chain of events leading to contraction;
- In β-cells of the pancreas, an action potential leads to the release of the hormone insulin.
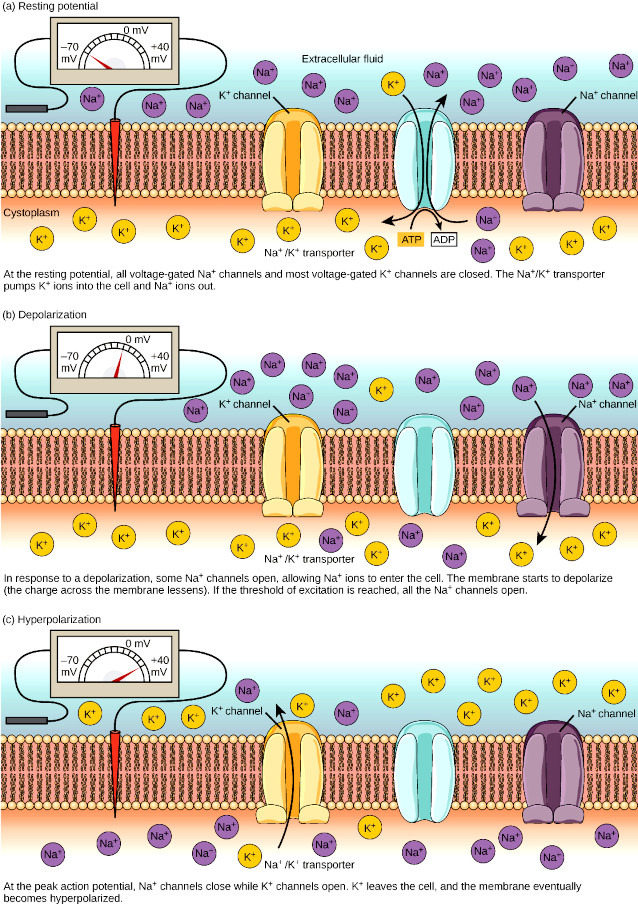
The complete cycle of neuron activation can be summarized in the following steps:
- Neurons can respond to a weak stimulus that causes a change in membrane potential. This is called a graded stimulus, because the stronger the stimulus, the larger the electrical change produced;
- However, if the stimulus is sufficiently strong to reach a threshold of -50 mV, an action membrane potential is initiated with a positive feedback loop that depolarizes the membrane to +30 mV. These action potentials carry the electrical signal along an axon, and
- are always of the same large amplitude of depolarization,
- are all-or-none response, meaning that can’t be graded,
- are actively propagated, and
- regenerate themselves as they travel
- After depolarization the membrane hyperpolarizes, becoming more negative before it reaches the resting potential of -70 mV.
Voltage-gated Na+ channels – The sodium ion plays the initial role in the development of the membrane action potential in the neuron. Sodium ions are mobilized through voltage-gated Na+ channels. During the cycle of neuron activation the voltage-gated Na+ channels can be found in three different states:
- Closed,
- Open, or
- Inactive.
The sequence of these events can be summarized as follows:
- At the resting membrane potential, voltage-gated Na+ channels are closed.
- When the stimulus reaches the threshold depolarization at the axon hillock the closed voltage-gated Na+ channels quickly open, meaning that open passage for Na+ that rapidly diffuses into cell causing the characteristic spike of action membrane potential.
- The voltage-gated Na+ channel closes immediately after, but as long as it is active it can be opened in response to a stronger stimulus. This is called the relative refractory period.
- However, when the voltage-gated Na+ channels become inactive, the passage of Na+ is completely blocked. This is called the absolute refractory period, which
- Makes the neuron unresponsive to another stimulus;
- Limits the frequency of action membrane potentials; and
- Ensures that action membrane potential does not move backwards toward the cell body.
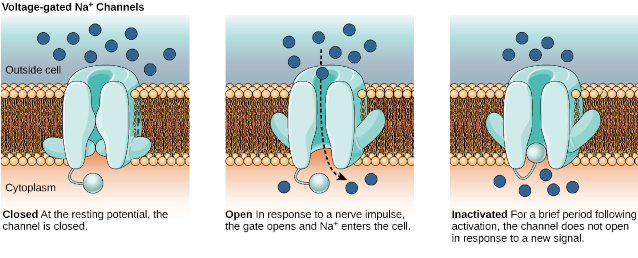
Voltage-gated K+ channels – The voltage-gated K+ channels also open at threshold depolarization of the axon hillock, but 1 ms later than the voltage-gated Na+ channels; shortly before that the voltage-gated Na+ channels close.
- When voltage-gated K+ channels open, more K+ leaves the cell than enters because the cytoplasm has a much higher K+ concentration than the extracellular fluid. This makes the intracellular side of the membrane more negative, causing repolarization;
- Following repolarization the voltage-gated K+ channels close slowly and may stay open until even after the membrane reach the resting membrane potential of -70mV;
- Furthermore, because the electrochemical potential of K+ is -90 mV, the ion continues to move out of the cell until the membrane becomes slightly hyperpolarized;
- When the voltage-gated K+ channels finally close, the membrane returns to resting potential, by which time the voltage-gated Na+ channels are also closed. Thus, both channels are shut when the membrane potential is near the resting potential.
Voltage-gated K+ channels open 1 ms later than voltage-gated Na+ channels, on average. In addition, the voltage-gated K+ channels close much more slowly than the voltage-gated Na+ channels. The difference in the time it takes for voltage-gated Na+ channels and voltage-gated K+ channels in response to threshold depolarization explains why repolarization occurs so quickly after depolarization.
5.2.3 Evolutionary Significance of the Voltage-Gated Channels
Most living things have voltage-gated ion channels, but it is only in the metazoa that you see a great diversity of kinds of voltage-gated ion channels. This is because voltage-gated ion channels are so central to the functioning of the nervous system, which relies on electrical signalling. The tiniest animals can get by without action potentials, but once the length of the body is greater than roughly one millimetre, graded potentials don’t have enough reach to send signals down longer axons – the amplification provided by action potentials becomes essential. As a result, specialized sets of voltage-gated ion channels involved in action potentials are a feature of nearly all animals.
5.2.4 Evolutionary Specializations for Speed of Signalling
As mentioned in the introduction of this chapter, the squid has a giant axon.
The squid has a giant axon for just one reason: speed[1]
The axon has a giant diameter because it’s critical for survival that a signal gets from the eyes to the mantle as fast as possible.
What is the limiting factor for the speed of an action potential down an axon? When an action potential happens at one place along an axon, it takes time for enough charge to build up further down the axon to trigger an action potential there. As a result, the speed at which an action potential travels down an axon is limited by the amount of time needed for sufficient charge to accumulate just ahead of the action potential. There are two ways to reduce that time:
- One way is to reduce the electrical resistance of the axon’s cytoplasm.
- The other way is to reduce the membrane capacitance, so that fewer ions are needed to produce a given voltage.
Increasing the diameter of the axon is a simple way to reduce electrical resistance. A bigger pipe allows faster flow of ions, like a bigger wire can carry more current. So it’s no surprise that giant axons have evolved independently several times in various invertebrate animals.
A simple way to reduce the membrane capacitance is to restrict access of ions to the extracellular surface. This is achieved by wrapping the axon in many layers of lipid bilayer in the form of a specialized glial cell called a Schwann cell. This is called myelination. Even though the myelinated segments of the axon do not need to have ion channels, and indeed have few ion channels, the electrical signal still needs to be re-amplified down the length of the axon. Regularly-spaced gaps interrupting the myelination, called nodes of Ranvier, accomplish this. These gaps in the myelination, the nodes of Ranvier, are the only areas of the axon that have enough voltage-gated Na+ channels to elicit an action potential. As a result, the conduction is saltatory (jumping), meaning the membrane action potential “jumps” from node to node.
To summarize saltatory conduction:
- In myelinated axons, ions can flow across the plasma membrane only at nodes where the myelin sheath is interrupted.
- Action potentials skip rapidly from node to node.
- Saltatory conduction allows thousands to millions of fast-transmitting axons to be packed into a relatively small diameter.
5.3 Signal Integration in a Neuron
Each neuron receives inputs from numerous other neurons, and neurons connect through a compartmentalized structure that permits the passage of chemical or electrical signals. This structure is called the synapse (Synapses were introduced in Chapters 3 and 4, and will be discussed in a lot more detail in Chapter 6), named after the close contact between the neuron and its target cell. Action potentials in the presynaptic neuron trigger a signal through the synapse to the postsynaptic neuron. The postsynaptic neuron then undergoes either a short-term change, typically a change in the membrane potential, or a longer-term change, often involving intracellular signalling cascades.
The connections among neurons define networks through which information travels and gets processed. Series of neurons interconnect and communicate through defined signalling pathways, forming “circuits” (see below). The neuron cell bodies often cluster together; These anatomical structures are called “ganglia”. The final point of a neural pathway is the regulated organ, where the synapse connects the terminal axon with a glandular or muscular cell.
Graded potentials in the dendrites, neuron cell body, and axon hillock provide an opportunity to integrate information from multiple signalling inputs. The graded potentials are controlled by ion channels, typically ligand-gated, in the postsynaptic membrane.
The dendrites and neuron body have relatively few voltage-gated Na+ channels (Table 5.2), allowing graded potentials to reach the axon hillock. Thus, signals from all the synapses on the dendrites and cell body converge in the hillock. The axon hillock has a number of specialized properties that make this region of the neuron capable of generating action potentials, including a much higher density of voltage-gated ion channels that in the rest of the neuron’s body (Table 5.2). If the graded potential reaches the threshold potential at the axon hillock, it triggers opening of those voltage-gated Na+ channels to start an action potential. Once triggered, the action potential travels the entire length of the axon.
| Neuron Body | Hillock | Node of Ranvier |
|---|---|---|
| 1 | 100-200 | 1000-2000 |
Signal inputs from the dendrites converging in the hillock can be excitatory or can be inhibitory, depending on the effect on the graded potential. This is because the presynaptic neurons can either generate stimulatory membrane potentials or inhibitory membrane potentials, depending on which neurotransmitters and receptors – often ligand-gated ion channels – are present at each synapse. Once the graded potential at the hillock reaches a triggering threshold, an action potential propagates through the rest of the axon. Action potentials in the presynaptic neuron end in a synapse where the presynaptic signal can be converted into one of two kinds of postsynaptic electrical signal, based on what effect the signal has on the postsynaptic graded potential:
- Excitatory Postsynaptic Potential (EPSP), and
- Inhibitory Postsynaptic Potential (IPSP)
Each neuron connects and receives impulses from as many as fifteen thousand other neurons. Initially the action membrane potentials are graded, and EPSP and IPSP are summed at the axon hillock. Neurons that receive sufficient excitatory impulses compared with inhibitory impulses generate action potentials.
- When the neuron receives equal inhibitory and excitatory potentials, no additional action potentials are triggered, because the inhibition cancels the excitation;
- When impulses are generated at different places of the neuron is termed spatial summation. If weak impulses are received simultaneously, by spatial summation the neuron may generate a sufficient graded potential to reach the firing threshold;
- When weak impulses are received at the same place in close succession is termed temporal summation. Thus, temporal summation of multiple weak impulses may also generate a sufficient graded potential to cause the neuron to fire.
EPSPs depolarize the membrane, bring the membrane closer to threshold potential, thereby making the neuron more likely to fire; IPSPs hyperpolarize the membrane, taking the membrane farther from the threshold potential, thereby making the neuron less likely to fire. Thus, the predominance of EPSPs or IPSPs at each synapse will determine if the stimulus is either stimulatory or inhibitory, and the predominance of EPSPs or IPSPs at the axon hillock will determine whether the neuron will start an action potential
Once an action potential has started, sequential opening and closing of voltage-gated Na+ channels directs a wave of depolarization from axon hillock to axon terminal. Inactivation of the voltage-gated Na+ channels after opening prevents backward movement toward the cell body, and the relatively slow closing of voltage-gated K+ channels compared with voltage-gated Na+ channels contributes to this effect Thus, membrane action potentials conduct signals unidirectionally and across long distances.
5.4 Circuits
When multiple neurons work together to process information, we often call that information processing path or network a “circuit”, by analogy with the electrical circuits in a computer. Of course, unlike the parts of a computer, neuronal circuits do not form actual electrical circuits, even though neuronal circuits process and conduct signal in the form of membrane voltages and action potentials. Neuronal circuits do not even necessarily form the looping paths implied by the word “circuits”.
Neuronal circuits can be extremely complex, though: Consider the gray matter of the brain (introduced in Chapter 2), especially brain regions such the neocortex and the olfactory bulb which store large amounts of information and perform complicated information processing. In gray matter, dendrites and synapses are densely packed together along with axon terminals and blood capillaries to form a mass called the neuropil (Greek, pil or pilos: felt)[2]. The neuropil comprises around 3/5 of the volume of gray matter, up to 4/5 in human neocortex. The remainder of the volume of gray matter is the cell bodies of neurons and glia, along with larger blood vessels. The observed proportion of neuropil in gray matter is close to the theoretical proportion that maximises connections among neurons while minimising the total length of the “wiring” (dendrite and axon length).
At the opposite extreme of neuronal circuit complexity are the neuronal circuits that carry out reflexes. Circuits that perform reflexes need to be simple in order to provide responses that are fast as possible. Reflexes are discussed in the next section.
Neurons can be classified into three broad categories based on their role in neuronal circuits:
- Afferent neurons – nerve cells that carry information toward the central nervous system, or farther centrally within the spinal cord and brain;
- Efferent neurons – nerve cells that carry information away from the brain or spinal cord, or away from the circuit;
- Interneurons – nerve cells that only participate in the local aspects of a circuit are called interneurons or local circuit neurons.
5.5 Reflexes
A reflex, or a reflex action, is an involuntary and almost instantaneous response to a stimulus. Because of the need for practically no delay before a response, processing time has to be minimised – information has to take the shortest possible path through the fewest neurons. As a result, reflex circuits are often relatively simple.
Most humans over the age of 2 have an amazing skill: the ability to walk on two feet. This feat of balance requires instant responses to tipping, and is possible due to the spinal reflex arc known as the “knee-jerk” or patellar reflex. The patellar reflex is an example of a basic neural circuit. Striking the patellar ligament in the knee stretches the quadriceps muscle of the leg, triggering a reflexive kick: The sensory signal caused by the stretch travels up afferent sensory neurons back to the spinal cord, and synapses with efferent motor neurons at the level of the fourth lumbar spinal nerve. The efferent signal back to the quadriceps muscle triggers its contraction, causing the leg to kick. A bipolar sensory neuron synapses directly on the motor neuron in the spinal cord, without interneurons in the pathway leading to contraction of the quadriceps muscle. Thus the path is direct, and can operate independently of the brain[3].
Reflexes are among the oldest and most noteworthy features of nervous systems. Reflexes exist in simple animals, such as flatworms, as well as complex mammals, such as humans. The response is quick and automatic. Many vertebrates will immediately cringe, jump, leap, or take flight in response to a loud noise, which could represent sudden danger. Animals that live in the water will reflexively dive deeper in response to a shadow overhead, which could represent passing of a predator. Infant primates have strong grasping reflexes that help them hold onto their mothers as they move. The patellar reflex is of clinical use to test of neural functionality and detection of sensory disorders and/or motor impulse transmission.
Integration of Signals
- Graded potentials – Neurons carry out integration by summing excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs).
- Summation – Summation pushes membrane potential of receiving cell toward or away from the action potential threshold.
No summation: The axon of Ex1 releases a neurotransmitter, which produces an EPSP in the postsynaptic cell. The membrane depolarizes, but not enough to reach threshold. If Ex1 input causes a new EPSP after the first EPSP has died down, it will be of the same magnitude as the first EPSP and no progression toward threshold has taken place—no summation has occurred.
Temporal summation: If instead, Ex1 input causes a new EPSP before the first EPSP has died down, the second EPSP will sum with the first and a greater depolarization will have taken place. This summation of two (or more) EPSPs produced by successive firing of a single presynaptic neuron over a short time is temporal summation. If the total depolarization achieved in this way reaches threshold, an action potential is produced in the postsynaptic neuron.
Spatial summation: The postsynaptic neuron may be brought to threshold by spatial summation, the summation of EPSPs produced by the simultaneous firing of two different excitatory presynaptic neurons, such as Ex1 and Ex2.
Summation resulting in cancellation: EPSPs and IPSPs can sum to cancel each other out. In the example, firing of the excitatory presynaptic neuron Ex1 alone produces an EPSP, firing of presynaptic inhibitory neuron In3 alone produces an IPSP, while firing of Ex1 and In3 simultaneously produces no change in the membrane potential.
5.6 Glossary
EPSP – Excitatory Postsynaptic Potential
IPSP – Inhibitory Postsynaptic Potential
ms – Milliseconds
mV – Millivolts
Ligand-gated channel – Ligand-gated channels open and close in response to signalling molecules, commonly neurotransmitters, that bind to the receptor domain of the channel.
Voltage-gated channels – Voltage-gated channels open and close in response to changes in voltage.
- We’re talking about giant axons in normal-sized squid here. Ironically, giant squid don’t have axons as large as normal-sized squid do. Presumably, if you’re big enough, the predators are afraid of you instead of the other way around. ↵
- In case you’re wondering about the white matter, it is mostly axons and glia and does not contain neuropil because it functions primarily to carry information between relatively distant regions of the brain. ↵
- If you distract the patient while performing the knee-jerk test. ↵

