6. Synapses and Neurotransmission
Chapter 6 – Synapses and Neurotransmission
6.1 Introduction
First, a reminder of what we learned in Chapters 4 and 5: Synapses are nerve connections where axons at nerve terminals meet a neuron, muscle or gland cells. A synapse is defined as a close contact between cells, specialised for communication of information between the two cells. Neurotransmission is defined as the transfer of information from a neuron to a target cell across the synapse. Neuromodulation is defined as the regulation of populations of neurons by chemical signals released outside the synapse by neurons.
At a synapse, the plasma membrane of an axon terminal comes into direct apposition with the plasma membrane of the target cell. The cell that is the source of the signal is called the presynaptic neuron while the receiving cell is called the postsynaptic cell. Both the presynaptic and the postsynaptic cells contain large arrays of molecular structures that connect membranes and participate in signalling.
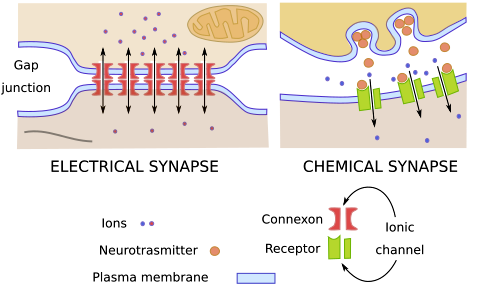
There are two different types of synapses, electrical synapses and chemical synapses. In electrical synapses, ions flow between the cytoplasms of the two cells through gap junctions, allowing an electrical signal to pass directly between the two cells. In chemical synapses, the signalling cell secretes a chemical signal called a neurotransmitter which is then detected by a receptor protein on the receiving cell.
6.2 Structure of a synapse
Electrical and chemical synapses have different anatomy. In electrical synapses, gap junction channels connect the presynaptic cell and postsynaptic cell in a distinctively close (2 to 4 nm) and uniform contact between the cells. Presynaptic neurons pass electrical current through the gap junctions, inducing voltage changes in the postsynaptic cell. The electrical current is in the form of a net flow of ions between the two cells.
In the chemical synapse, there is a wider space between the two cells, called the synaptic cleft: The presynaptic neuron releases the neurotransmitter by exocytosis, which diffuses into the synaptic cleft adjacent to the receiving neuron.
The chemical signalling molecules secreted by neurons are called neurotransmitters. Neurotransmitters bind to receptors in the postsynaptic cell membrane. As you learned in Chapter 3, many of the postsynaptic receptors are ligand-gated ion channels, and many of the other postsynaptic receptors are G protein coupled receptors (GPCRs). Many of the most important neurotransmitters are made in nerve terminals from a simple, abundant precursor, such as an amino acid. Neurotransmitters are packaged into synaptic vesicles on the presynaptic side of a synapse and are released when a nerve impulse reaches the synapse. By binding to receptors and thereby affecting the opening and closing of ion channels, the released neurotransmitters affect the membrane potential of the connecting cell. The effect can be either inhibitory, by hyperpolarising the membrane, or excitatory, by partially depolarising the membrane. However, neurotransmitters are also released following graded electrical potentials into the synaptic cleft as well as secreted at a low level baseline in the absence of electrical stimulation.
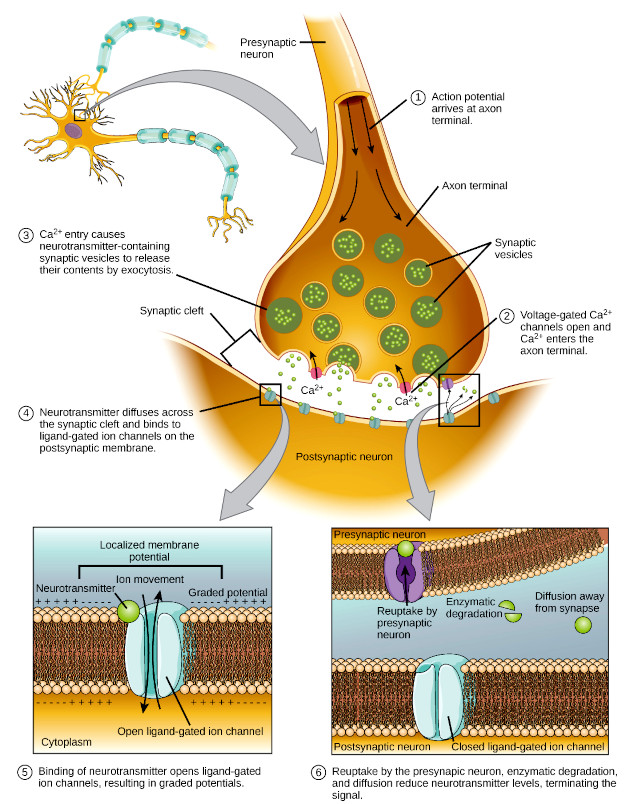
The arrival of an action potential at the axon terminal rapidly generates a local influx of calcium ions (Ca2+) into the presynaptic cytoplasm through voltage-gated Ca2+ channels. Calcium ions then bind to synaptotagmin, a Ca2+ sensor protein embedded in the membrane of presynaptic vesicles, allowing the vesicles to rapidly fuse with the presynaptic membrane, which releases the neurotransmitter by exocytosis. The exocytotic membrane fused to the cell membrane is soon afterwards reintroduced into the cytoplasm by endocytosis and recycled for the formation of a new vesicle filled with the neurotransmitter.
6.3 Neurotransmitters
Over fifty different substances that act as neurotransmitters have been found in the animal body. They are commonly divided into five classes, according to their size, structure, and function (Table 6.1).
| Class | Neurotransmitter | Functional Class | Secretion Site | |
|---|---|---|---|---|
| Choline-derived | Acetylcholine | Excitatory to vertebrate skeletal muscles; Excitatory or inhibitory at other sites. |
CNS; PNS, neuromuscular junction in vertebrates | |
| Biogenic amines | Norepinephrine (noradrenaline) and Epinephrine (adrenaline) |
Excitatory or inhibitory | CNS; PNS | |
| Dopamine | Usually excitatory, but may be inhibitory at some sites | CNS, PNS | ||
| Histamine | Excitatory | GI tract, CNS, PNS | ||
| Serotonin | Excitatory or inhibitory | GI tract, platelets, CNS. | ||
| Octopamine | Excitatory or inhibitory | invertebrates only, mostly CNS | ||
| Tyramine | Excitatory or inhibitory | invertebrates only, mostly CNS | ||
| Amino acids | GABA (γ-aminobutryric acid) | Inhibitory | CNS in vertebrates; mostly neuromuscular junction (PNS) in many invertebrates | |
| Glycine | Inhibitory | CNS | ||
| Glutamate and Aspartate | Excitatory | CNS; neuromuscular junction (PNS) in some invertebrates | ||
| Neuropeptides | Substance-P | Excitatory | CNS; PNS | |
| Enkephalines | Generally inhibitory | CNS | ||
| Gases | Nitric oxide | Vasodilator | Blood vessels | |
| Hydrogen sulphide | Vasodilator | Blood vessels | ||
| Carbon monoxide | Blood vessel relaxant | Blood vessels |
Research on natural compounds that mimic the effect of neurotransmitters has benefitted the study of signalling receptors in the nervous system.
A compound that binds to a receptor is termed a ligand for the receptor. Ligands that activate the receptors are called agonists, whereas ligands that inhibit a receptor are called antagonists. Some compounds from natural sources that are ligands of the cholinergic receptors were used to characterize the effects and mechanism of action of acetylcholine. They include pilocarpine, atropine, nicotine, muscarine, and curare.
Each of these compounds has a story to tell about how humans have used chemicals found in nature. They also have a story to tell about acetylcholine and its receptors.
Atropine is an alkaloid extracted from deadly nightshade (Atropa belladonna). It is a competitive antagonist of the muscarinic ACh receptors. Atropine increases heart rate and reduces salivation and other secretions. Topical use of atropine induces mydriasis – dilation of the pupil – by blocking of the circular papillary sphincter muscle that dilates the pupil of the eye.

Curare is a common name for various plant extracts used as a paralyzing poison by indigenous peoples of the Caribbean and tropical South America. They used arrows or blowgun darts dipped in curare to shoot game. The animals die by asphyxiation due to paralysis of the respiratory muscles: Curare inhibits the nicotinic acetylcholine receptor (nAChR) at the neuromuscular junction.

Muscarine is a quaternary ammonium salt found in the fly agaric mushroom, Amanita muscaria. Fly agaric is highly poisonous, and its name comes from its use in the past to kill houseflies. Muscarine binds and activates a cholinergic G protein-coupled metabotropic receptor.
Nicotine is an alkaloid isolated from tobacco, Nicotiana tabacum. Nicotine is the mind-altering, highly addictive drug present in tobacco smoke. Nicotine binds and activates a cholinergic ligand-activated ion channel receptor.
Pilocarpine is an alkaloid obtained from the leaves of tropical South American shrubs in the genus Pilocarpus. Pilocarpine is a non-selective muscarinic cholinergic receptor M3 agonist. It stimulates the secretion of large amounts of saliva and fluids secreted by the sweat glands in the skin.
6.3.1 Acetylcholine (ACh)
ACh is a polyatomic cation made from choline[1] and acetyl-CoA by choline acetyltransferase, an enzyme abundant in certain neurons. Neurons that produce ACh are called cholinergic neurons. Acetylcholinesterase in the synaptic cleft converts ACh into the inactive metabolites choline and acetate. ACh is one of most widespread neurotransmitters in both the CNS and in the PNS.
ACh-secreting neurons and associated neurons form a network called the cholinergic system, that causes anti-excitatory actions and plays a role in sensory perceptions, sustaining attention, arousal and reward.
ACh is the main neurotransmitter of the ANS, and is released in the following sites:
- preganglionic fibres of the parasympathetic and sympathetic divisions;
- post-ganglionic parasympathetic fibres;
- preganglionic sympathetic fibres of the adrenal medulla, which on stimulation by ACh releases epinephrine and norepinephrine;
- some postganglionic sympathetic fibres such as the sudomotor neurons of the sweat glands.
ACh is the only neurotransmitter of the motor division of the somatic nervous system (SNS). It acts as an excitatory neurotransmitter at neuromuscular junctions in skeletal muscle. ACh binds to nicotinic receptors (described below) that open membrane ligand-gated sodium channels and initiate a sequence of steps leading to muscle contraction.
In the heart, ACh lowers the heart rate. By acting through a muscarinic receptor (described below) in the non-voluntary fibres of the heart, ACh inhibits contraction of the cardiac muscle.

ACh Receptors ACh binds to two distinct classes of receptors: the nicotinic receptor and the muscarinic receptor, named for the ligands used to distinguish them: the nicotinic receptor binds to nicotine, an alkaloid isolated from the tobacco plant Nicotiana tabacum, while the muscarinic receptor binds muscarine, a toxin from the fungus Amanita muscaria. Both classes of receptors are termed cholinergic receptors, and the neurons containing ACh are termed cholinergic fibres, including the preganglionic sympathetic fibres and the preganglionic and post-ganglionic parasympathetic fibres.
Nicotinic Receptors – also termed ionotropic receptors, are ligand-gated membrane channels (LGIC; described in Chapter 3) that respond very rapidly to ACh allowing passage of sodium, potassium, and calcium ions into the cell. There are two main types of nicotinic receptors:
- Nicotinic muscular type, expressed exclusively in the neuromuscular plate; and
- Nicotinic neuronal type, expressed mainly in parasympathetic and sympathetic ganglia of the ANS and in the CNS.
In the postganglionic neuron, the nicotinic receptors are responsible for the initial fast depolarization or fast excitatory postsynaptic potential (Fast EPSP).
A shocking discovery
Torpedo californica, or Tetronarce californica, is a species of electric ray found in the northeastern Pacific Ocean. The muscle tissues of electric rays have evolved into an electric organ with a structural organization similar to the mammalian neuromuscular junction (NMJ). This tissue is organized into two kidney-shaped glands located on either side of the head that can produce an electrical shock strong enough to knock down a human adult. The electric organs Torpedo californica contain an abundance of nicotinic cholinergic receptors and acetylcholinesterase, the enzyme that degrades the neurotransmitter acetylcholine. The acetylcholine receptor from Torpedo californica was the first neurotransmitter receptor to be isolated and sequenced*.

Electric organs contain electrocytes (Chapter 5) that temporarily store electrical energy in the form of an electrostatic field. To discharge the electrocytes electric eel uses a pacemaker located in the nucleus of pacemaker neurons. When an electric eel spots its prey, the pacemaker neurons fire a release of acetylcholine from electromotor neurons to the electrocytes, resulting in an electric discharge. Each electrocyte can only generate tens of millivolts, but because many cells are stacked together the total voltage of the electric shock can be 45 volts.
*Hucho, F., Layer, P., Kiefer, H. R. and Bandini, G. Photoaffinity, labeling and quaternary structure of the acetylcholine receptor from Torpedo californica (subunit function/toxin binding site/molecular weight/postsynaptic membrane structure). Proc. Natl. Acad. Sci. USA Vol.73, No.8, pp. 2624-2628, August 1976.
Medical conditions illustrate the role of nicotinic cholinergic receptor
Myasthenia gravis (Greek, mûs: muscle; asthéneia, weakness; Latin, gravis: serious) is a neuromuscular disease caused by circulating antibodies against the nicotinic acetylcholine receptors. These antibodies block the ability of acetylcholine to bind to its receptors at the postsynaptic neuromuscular junction in the muscle. By blocking the ability of the neurotransmitter acetylcholine to bind to these receptors, these antibodies keep motor neurons from signalling the muscle to contract causing muscle weakness and fatigue.
Intoxication by organophosphates. Organophosphates are esters of phosphoric acid, used as the active ingredient of many insecticides, herbicides and nerve agents. OPs are one of the most common causes of poisoning worldwide. The United States Environmental Protection Agency lists OPs as highly toxic to bees, wildlife, and humans.
Organophosphates are irreversible inhibitors of acetylcholinesterase, the enzyme that inactivates ACh in the synaptic cleft. Acetylcholinesterase cleaves OPs, leaving the phosphoryl group in the esteric site of the enzyme. The phosphoryl group is hydrolyzed very slowly (in the order of days) and can become covalently bound to acetylcholinesterase, leading to a pathological excess of acetylcholine in the body and subsequently inhibiting its action on nerve cells by down-regulation of the nicotinic cholinergic receptor. Irreversible inhibition of acetylcholinesterase may lead to muscular paralysis, convulsions, bronchial constriction and death by asphyxiation. Toxicity of organophosphates is not limited to the acute phase, since chronic effects have long been described. As acetylcholine has a crucial role in the brain’s development, organophosphates have dramatic effects on developing organisms even from low levels of exposure.
Muscarinic Receptors – also termed metabotropic receptors, are members of the family of the G protein coupled receptors (GPCRs, described in Chapter 3). Upon binding to ACh, muscarinic receptors remain active over a longer time frame than nicotinic receptors, mediating mostly inhibitory effects. Muscarinic receptors are present in both the CNS and the PNS, and additionally the heart, lungs, upper gastrointestinal tract, and sweat glands. Based on their association with the G proteins (described in Chapter 4) and on their intracellular effects, five subtypes of muscarinic receptors, have been described, numbered M1 to M5 (Table 6.2).
| Receptor Type | Mechanism of Action | Functions |
|---|---|---|
| M1 |
|
|
| M2 |
|
|
| M3 |
|
|
| M4 |
|
|
| M5 |
|
|
6.3.2 Biogenic Amines
Biogenic amine neurotransmitters include the three catecholamines, norepinephrine, epinephrine, dopamine, histamine, serotonin, octopamine, and tyramine. Norepinephrine, epinephrine, and dopamine are called catecholamines because of their structure. Epinephrine is also known as adrenalin, while norepinephrine is also known as noradrenalin.
Norepinephrine – derived from the amino acids phenylalanine and tyrosine. Norepinephrine has a dual role, is mainly a neurotransmitter although it works as a hormone as well. Norepinephrine as a neurotransmitter is released by the sympathetic neurons, mainly regulating the heart and the muscular vasculature. Norepinephrine also affects sleep, wakefulness, attention and feeding behaviour. Norepinephrine as a hormone is secreted by the chromaffin cells of the adrenal medulla and is involved in stress, affecting parts of the brain controlling response and attention. Norepinephrine is secreted during the fight-or-flight response increasing heart rate and contractions, and blood flow to the skeletal muscles. It also increases oxygen supply to the brain and glycogenolysis releasing glucose from stores.
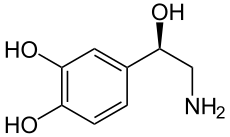
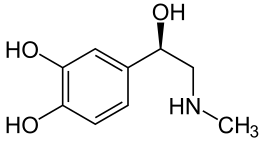
Epinephrine – also derived from phenylalanine and tyrosine. Epinephrine has a dual role, although its hormonal activity is more predominant. Epinephrine is secreted by the chromaffin cells of the adrenal medulla under conditions of stress such as the fight-or-flight response. Its actions are similar to the hormonal effects of norephinephrine, increasing heart rate and contractions, blood flow to the skeletal muscles, oxygen supply to the brain and glycogenolysis. As a neurotransmitter, epinephrine is present at low levels in the brain of mammals.
Adrenergic Receptors The receptors for norepinephrine and epinephrine are called adrenergic receptors. Both compounds bind and activates the adrenergic receptors, although not necessary driving the same effects, which depends on the type of receptor and tissue localization. There are two main groups of adrenergic receptors, termed adrenergic-α (Table 6.3) and adrenergic-β (Table 6.4), with several subtypes in each group.
| Adrenergic-α Receptor | Relative Potencies of Norepinephrine and Epinephrine | Mechanism of Action | Functions |
|---|---|---|---|
| Adrenergic-α1 | Norepinephrine > Epinephrine | Coupled to Gq:
|
Smooth muscle contraction in:
Metabolic actions:
|
| Adrenergic-α2 | Epinephrine ≥ Norepinephrine | Coupled to Gi:
|
Inhibit:
Stimulate:
Mixed effects on smooth muscle |
| Adrenergic-β Receptors | Relative Potencies of of Norepinephrine and Epinephrine | Mechanism of Action | Function |
|---|---|---|---|
| Adrenergic-β1 | Epinephrine = Norepinephrine | Coupled to Gs:
|
Stimulate:
|
| Adrenergic-β2 | Epinephrine >> Norepinephrine | Coupled to both Gs and Gi | Relax smooth muscle:
Stimulate:
|
| Adrenergic-β3 | Epinephrine > Norepinephrine | Coupled to Gs:
|
Abundant in adipose tissue Stimulate lipolysis |
Overall, the adrenergic receptors are expressed in a variety of tissues, mediating the effect of the sympathetic ANS. The final effects of the adrenergic receptors depend on which organ they are in, the regulatory needs of the tissue, and the physiological status of the animal.
Dopamine – is also a catecholamine with a double role, as a neurotransmitter and a endocrine/paracrine chemical messenger. Neurons secreting dopamine are called dopaminergic neurons. As a neurotransmitter. dopamine plays a significant role in the brain, regulating behaviour and cognition[2]. Brain functions requiring dopaminergic neurons include reward-driven learning, voluntary movement, motivation, sleep, dreaming, mood, attention, and working memory[3].
Dopaminergic neurons are present in the arcuate nucleus of the hypothalamus, which secretes dopamine into the blood vessels of the median eminence that irrigates the pituitary gland. In the pituitary gland, dopamine inhibits the lactotroph cells to produce prolactin, a protein hormone involved in lactation (milk production) and sexual gratification. Dopamine is believed to be the hypothalamic prolactin-inhibiting factor (PIF), also called prolactin-inhibiting hormone (PIH) or prolactostatin.
Much of information about the role of dopamine in the endocrine system emerged from studies using drugs that reduce dopamine secretion. Drugs that reduce dopamine secretion have often been used as antipsychotics. In women, pharmacological reduction of dopamine lowers levels of estradiol and progesterone. Pharmacological reduction of dopamine can also lead to hyperprolactinemia, which causes amenorrhea, abnormal ovarian function, loss of libido, false positive pregnancy tests, and long-term risk of osteoporosis. In men, antipsychotic drugs cause gynaecomastia, lactation, impotence, loss of libido, and reduction in spermatogenesis.
Dopamine binds to five main classes of G proteins coupled receptors, grouped into two families, the D1-like and the D2-like:
- The D1 family that includes the D1 and D5 receptors coupled to Gs. As mentioned in Chaper 4, Gs activates adenylyl cyclase, increasing the intracellular cAMP level. Through the D1 family of receptors, dopamine regulates opening of membrane ion channels, allowing mobilization of Na+ and K+.
- The D2 family includes the D2, D3, and D4 receptors, coupled to Gi and Gq, which inhibit adenylate cyclase, decreasing intracellular cAMP. Therefore, the D2-like receptors usually mediate the inhibitory actions of dopamine, decreasing the generation of membrane action potentials.
Histamine – is a vasoactive amine neurotransmitter, derived from the decarboxylation of the amino acid histidine. Mast cells[4] release histamine in response to tissue damage and allergic reactions. Histamine is involved in local immune responses as well as a neurotransmitter regulating functions in the CNS and the gastrointestinal tract.
In the immune system, histamine is produced by the basophils and by mast cells of the connective tissues, triggering the inflammatory response to pathogens. Histamine increases the permeability of the capillaries to white blood cells and some proteins, including the immunoglobulins, letting them reach the infected tissue. In the gastrointestinal tract, the enterochromaffin-like cells of the stomach release histamine that stimulates secretion of HCl.
In the CNS, histamine mediates reactions related to arousal and attention. Histamine binds to four types of G protein coupled receptors (Table 6.5).
| Type | Location | Function |
|---|---|---|
| H1 | Smooth muscle, endothelium and CNS | Contraction of the bronchial smooth muscles, vasodilatation, separation of endothelial cells causing pain and itching, motion sickness and sleep |
| H2 | Parietal cells of the stomach and vascular smooth muscle | Vasodilatation |
| H3 | CNS and PNS | Decrease release of histamine, acetylcholine and norephinephrine |
| H4 | Basophils, bone marrow, thymus, small intestine, and colon | Mediate cell chemotaxis[5] |
Serotonin – also called 5-hydroxytryptamine (5-HT), is a neurotransmitter derived from the amino acid tryptophan. Serotonin is found in three main sites in the animal body: the gastrointestinal (GI) tract, platelets, and the CNS.
- The enterochromaffin cells[6] of the gastrointestinal tract produce serotonin, which regulates intestinal movements.
- Platelets take and store serotonin secreted from the enterochromaffin cells. Platelets bind to a blood clot in injured tissues and pour serotonin out to participate in the process of hemostasis serving as vasoconstrictor.
- The serotonergic neurons of the CNS produce serotonin, which regulates various biological and neurological processes, including aggression, anxiety, appetite, cognition, learning, memory, mood, nausea, sleep, and thermoregulation. These effects of serotonin may not necessarily be direct effects since it also regulates the release of other neurotransmitters including:
- Glutamate, γ-aminobutyric (GABA), dopamine, epinephrine, norepinephrine and acetylcholine; and
- Hormones, such as oxytocin, prolactin, vasopressin, cortisol, and corticortropin, among others.
The serotonin receptors[7] belong to the family of GPCR and ligand-gated ion channels (LGIC) receptors found in the CNS and peripheral NS. The serotonin receptors mediate both the excitatory and inhibitory effects of the neurotransmitter. Seven types of serotonin receptors have been described (Table 6.6).
| Serotonin Receptor | Mechanism of Action | Effects |
|---|---|---|
| 5-HT1 | Coupled to Gi/Go, inhibits adenylate cyclase and reduces cAMP. | Inhibitory |
| 5-HT2 | Coupled to Gq/G11. IP3 and DAG. | Excitatory |
| 5-HT3 | Mobilizes Na+ and K+ through LGIC, causing plasma membrane depolarization. | Excitatory |
| 5-HT4 | Coupled to Gs, activates adenylate cyclase and raises cAMP levels. | Excitatory |
| 5-HT5 | Coupled to Gi/Go, inhibits adenylate cyclase and reduces cAMP levels. | Inhibitory |
| 5-HT6 | Coupled to Gs, activates adenylate cyclase and raises cAMP levels. | Excitatory |
| 5-HT7 | Coupled to Gs, activates adenylate cyclase and raises cAMP levels. | Excitatory |
6.3.3 Amino Acid Neurotransmitters
Amino acid neurotransmitters are classified into two main groups, inhibitory amino acids (IAA) and excitatory amino acids (EAA).
- IAA are depressors of the post-synaptic cells and include gamma aminobutyric acid (γ-aminobutyric acid or GABA), glycine, β-alanine, and taurine;
- EAA include L-glutamate, L-aspartate, L-cysteine, and L-homocysteine.
GABA – γ-aminobutyric acid is an amino acid not present in proteins. Neurons that produce GABA are called gabaergic neurons. GABA is the main inhibitory neurotransmitter in the CNS and the main regulator of muscle tone in mammals. GABA binds to specific transmembrane receptors in pre- and post- synaptic membranes. Two general classes of GABA receptors have been described:
- GABAA – a ligand-gated ion channel; and
- GABAB – a G protein-coupled metabotropic receptor that regulates ion channels.
GABA-gated ion channels typically have an inhibitory effect: GABA binding opens ion channels to allow the flow of either negatively charged chloride ions into the cells or positively charged potassium ions out of the cell, resulting in hyperpolarization (a negative change in the membrane potential). The direction of the chloride flow determines the polarization status of the neuron’s membrane.
- When net chloride flows out of the cell, GABA is excitatory or depolarizing, makes the voltage across the cell’s membrane less negative;
- When the net chloride flows into the cell, GABA is inhibitory or hyperpolarizing, makes the voltage across the cell’s membrane more negative;
- When the net flow of chloride is close to zero, GABA has no direct effect on the cell’s membrane potential. This action of GABA is termed shunting inhibition, which means that any coincident synaptic input is minimized by a reduction of the electrical resistance of the membrane.
The molecular mechanisms controlling concentration of chloride inside the cells change during development. These changes result in a developmental switch in the way the direction chloride flows, changing the functional role of GABA between the neonatal and adult stages, from excitatory to inhibitory.
Glycine – is an inhibitory amino acid neurotransmitter in the spinal cord, brain and retina. Glycine binds to ionotropic receptors causing chloride ions to enter the cells triggering an inhibitory postsynaptic potential (IPSP). Glycine is a co-agonist of glutamate in the NMDA receptor (described below).
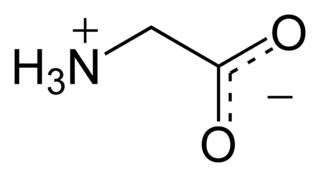
Glutamate – glutamic acid is the most abundant excitatory neurotransmitter in the nervous system of vertebrates. It is the brain’s main excitatory neurotransmitter, and also a precursor for GABA, the brain’s main inhibitory neurotransmitter (see above). One of the major functions of glutamate is modulation of synaptic plasticity, a vital property of the brain for the process of memory and learning. Thus, glutamate is involved in cognitive functions that require memory formation and learning.
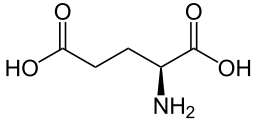
Glutamate mediates its effects through ionotropic (iGluR) and metabotropic (mGluR) receptors found in the glial cells and in neurons of the CNS. Ionotropic receptors tend to be faster in relaying information, but metabotropic ones are associated with more prolonged stimuli. Ionotropic glutamate receptors allow the flow of K+, Na+ and sometimes Ca2+ in response to glutamate binding. Upon glutamate binding, an ion channel opens, allowing ion flow generating an EPSC that trigger and action potential in the postsynaptic neuron. Metabotropic glutamate receptors (mGluRs) are coupled to G proteins that activate ion channels on the plasma membrane. The mGluRs mainly mediate inhibition of adenylate cyclase reducing the activity of the cAMP-dependent pathway.
Mammalian glutamate receptors are classified based on their ability to bind a variety of different pharmacological agents that mimic the action of glutamate. Thus, at least three groups of iGluR and three groups of mGluR have been characterized. The iGluR include the NMDA, the kainite, and the AMPA receptors.
NMDA Receptor – NMDA stands for N-Methyl-D-aspartic acid – an amino acid derivative that mimics the action of the natural ligand, glutamate. But unlike glutamate, NMDA only binds to and regulates the NMDA receptor and has no effect on any other glutamate receptor. The NMDA receptor is different that the other glutamate receptors in two ways, first, it is both a membrane ligand-gated and a membrane voltage-gated, and second, it requires co-activation by two ligands, glutamate and either d-serine or glycine,
- Because it depends on voltage changes to be activated, the ion channel of the NMDA receptor can be blocked by extracellular Mg2+ ions. Thus, a positive change in membrane potential expels the Mg2+ that block the channel from outside of the membrane. These changes facilitate the flow of Na+ and small amounts of Ca2+ ions into the cell and K+ out of the cell. Calcium flux through NMDA receptor is thought to be critical in synaptic plasticity, a cellular mechanism for learning and memory.
- Activation by glutamate requires either d-serine or glycine as co-agonists for the efficient opening of the ion channel. d-serine co-agonizes the NMDA receptor with greater potency than glycine.
NMDA receptors can be overactivated after withdrawal of alcohol consumption, causing agitation and, epileptiform seizures.
Kainate Receptor – Kainate receptors respond to glutamate and to kainic acid, a drug isolated from the red alga Digenea simplex. Postsynaptic kainate receptors are involved in excitatory neurotransmission, while presynaptic kainate receptors are involved in inhibitory neurotransmission by modulating release of the inhibitory neurotransmitter GABA.
AMPA Receptor – AMPA stands for 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid, a weak organic acid with a phosphonic group that also mimics the effects of glutamate. It is one of the primary degradation products of the herbicide glyphosate. AMPA has low toxicity, which is comparable to that of glyphosate and it is therefore considered to be of no greater toxicological concern than glyphosate itself.
The mGluR include the glutamate receptors L-AP4, ACPD, and LQA. L-AP4 is a compound selective agonist of the different subtypes of mGluR. ACPD stands for 1-amino-1,3-dicarboxycyclopentane, a chemical compound that binds and activates the mGluRv with no effect on the iGluR. ACPD induces convulsions in neonatal rats. Quisqualic acid is an agonist for both AMPA receptors and a subtype of mGluR.
6.3.4 Neuropeptides
Substance P is a neuropeptide that belongs to a family of neuropeptides called tachykinins, produced from a common polyprotein precursor. Substance P function as a neurotransmitter as well as a neuromodulator. The terminals of sensory nerves of the brain and spinal cord release substance P in response to inflammatory processes and pain.
Substance P mediates its effects through a specific GPCR termed neurokinin 1 receptor that belong to subfamily of tachykinin receptors. Substance P and the neurokinin 1 receptors are distributed in regions of the brain that regulate emotions, including the hypothalamus and amygdala. Both the peptide and the receptor are found in close association with serotonin and neurons secreting norepinephrine.
Substance P is a component of the sensory system that transmits pain information to the CNS; it coexists with the excitatory neurotransmitter glutamate as primary afferents that respond to painful stimulation. This activity is initiated by nociceptors, also termed pain receptors, which can detect mechanical, thermal or chemical changes. Once stimulated, nociceptors transmit a signal along the CNS. In addition, substance P has been associated with the regulation of mood disorders, anxiety, stress, neurogenesis, respiratory rhythm, neurotoxicity and nausea.
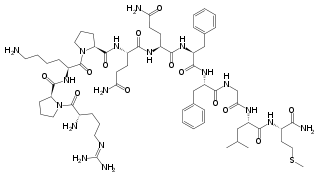
Enkephalins are pentapeptides, endogenous ligands of the delta-opioid (δ-opioid) receptor. Activation of δ-opioid receptors produces some analgesia although less than that of the mu-opioid (μ-opioid) agonists, such as the opium alkaloid morphine.
Two forms of endogenous enkephalins are found in the CNS, metenkephalin (also called opioid growth factor) with opioid effects of short duration, and leuenkephalin that has agonistic actions at both the δ-opioid and μ-opioid receptors. Metenkephalin is found mainly in the adrenal medulla and throughout the CNS, including the dorsal horn of the spinal cord, and in the periphery innervating pelvic viscera.
6.3.5 Gasotransmitters
Gasotransmitters are small molecules with no net electrical charge, therefore they freely cross membranes and do not require a receptor on the cell surface to mediate their effects. Gasotransmitters are endogenous (made inside the body) signalling molecules that transmit information in the animal body. The name “gasotransmitter” is due to the fact that small, nonionic molecules tend to be gases at room temperature and atmospheric pressure. However, the gasotransmitters made endogenously (inside the body) by enzymes within cells are made at low enough concentrations to remain dissolved. They can function as endocrine, paracrine, and autocrine regulators. Characterized gasotransmitters in the animal body include nitric oxide, carbon monoxide, and hydrogen sulphide.
Nitric oxide (NO) is a free radical[8] signalling molecule involved in many physiological and pathological processes. Nitric oxideis also known as nitrogen oxide or nitrogen monoxide. It is a metabolic bioproduct in almost all types of organisms, ranging from bacteria to plants, fungi, and animal cells and is a key vertebrate biological messenger.
NO is made endogenously from the amino acid L-arginine, oxygen and NADPH by various nitric oxide synthase (NOS). In the animal body NO is generated by the endothelium of blood vessels and by phagocytes, including monocytes, macrophages and neutrophils, as part of the immune response system. When secreted as part of an immune response, NO as free radicals is toxic to bacteria causing DNA damage. NO is highly reactive with a lifetime of a few seconds. It diffuses freely across membranes for which is considered a paracrine and autocrine signalling molecule.
NO is also known as the “endothelium-derived relaxing factor” or “EDRF”, because is a powerful vasodilator. It has only a short half-life of a few seconds in circulation. The endothelium generates NO to relax the surrounding smooth muscle, resulting in vasodilation and increased blood flow. This is the reason why NO is elevated in populations living at high altitudes causing vasodilation of the pulmonary vasculature to avoid hypoxia.
Classic drugs used as vasodilators, such as nitroglycerine and amyl nitrite, are converted to nitric oxide in the body. The vasoactive effects of NO also affect the penile vasculature producing erection.
NO activates the soluble guanylate cyclase, a heterodimeric enzyme that catalyze the formation of cyclic GMP from GTP. Cyclic GMP (cGMP) activates protein kinase G, which stimulates reuptake of Ca2+ and opens calcium-activated potassium channels. The fall in concentration of Ca2+ ensures that the myosin light chain kinase (MLCK) no longer phosphorylates the myosin molecule and thereby stopping the crossbridge cycle (Chapter 7), leading to relaxation of the smooth muscle cell.
The drug sildenafil (sold as Viagra®) stimulates erections primarily by enhancing signalling through the nitric oxide pathway causing vasodilation in the penis. Specifically, sildenafil binds to a cGMP phosphodiesterase and inhibits it, preventing hydrolysis of cGMP. As a result, in the presence of the drug, only a small amount of NO-activated guanylate cyclase activity is needed in order to attain maximal cGMP levels.
Hydrogen sulfide (H2S) is a signalling gas produced from the amino acid cysteine by cystathionine beta-synthase and cystathionine gamma-lyase. It acts as a relaxant of smooth muscle and as a vasodilator. H2S is also active in the brain, where it increases the response of the NMDA receptor and facilitates long-term neural potentiation, which is a process involved in the formation of memory. H2S is converted into sulfite in the mitochondria by thiosulfate reductase, and is further oxidized by sulfite oxidase to thiosulfate and sulfate. These breakdon products are finally excreted in the urine.
Like NO, H2S relaxes blood vessels, but their mechanisms of action are different. H2S activates ATP-sensitive potassium channels in smooth muscle, while NO mediates its effects by activating guanylyl cyclase. Due to these effects, similar to NO, H2S is recognized as potentially protecting against cardiovascular diseases. It is believed that the cardio protective role of garlic is caused by catabolism of the polysulfide group of an organosulfur compound termed allicin that is converted into H2S.
It is not clear how the vessel-relaxing responsibilities are shared between NO and H2S. There is some evidence suggesting that NO relaxes large vessels, while H2S relaxes smaller blood vessels. It is believed that the vasodilatatory effects of both gases are mutually dependent due to crosstalk between NO and H2S signalling pathways in the cell. Like NO, H2S is involved in the relaxation of smooth muscle that causes erection of the penis.
Carbon Monoxide (CO) is a gaseous neurotransmitter produced from heme, a product of hemoglobin breakdown, by the enzymes heme oxygenase-1 and heme oxygenase-2. This process produces a certain amount of carboxyhemoglobin in a normal individual even if do not inhale any CO. CO is as a signalling molecule in the neuronal system, involved in the regulation of neurotransmitters and neuropeptide release, learning and memory, response to odour, adaptation, modulation of inflammatory responses and muscle relaxation.
6.4 Summary
- Synapses are junctions where axon terminals meet other cells.
- Transfer of information across the synapse is called neurotransmission.
- Neuromodulation is a process by which a given neuron uses several different neurotransmitters to regulate diverse populations of neurons.
- Neurotransmitters are commonly divided into five classes, according to their size, structure and function.
- ACh is one of most widespread neurotransmitters in both the CNS and in the PNS and the only neurotransmitter in the motor division of the SNS.
- In the ANS, ACh is released in the preganglionic fibers of the parasympathetic and sympathetic divisions, in the post-ganglionic parasympathetic fibres, preganglionic sympathetic fibres to the adrenal medulla, and some postganglionic sympathetic fibres such as the sudomotor neurons to sweat glands.
- ACh binds to two distinct classes of receptors, the nicotinic receptor and the muscarinic receptor.
- Nicotinic receptors are ligand-gated membrane channels.
- Muscarinic receptors are G protein coupled receptors.
- Biogenic amine neurotransmitters include the three catecholamines, norepinephrine also known as noradrenalin, epinephrine also known as adrenalin, and dopamine, histamine, and serotonin.
- The receptors for norepinephrine and epinephrine are termed adrenergic receptors.
- Dopamine is also a catecholamine with a double role as a neurotransmitter and hormone.
- As a neurotransmitter dopamine regulates behaviour and cognition, including reward-driven learning voluntary movement, motivation, sleep, dreaming, mood, attention and working memory.
- Dopamine is secreted into the blood vessels that irrigate the pituitary, inhibiting prolactin production.
- Histamine is a vasoactive amine neurotransmitter, released from mast cells in response to tissue damage and allergic reactions.
- Histamine is involved in local immune responses as well as a neurotransmitter regulating functions in the CNS and the gastrointestinal tract.
- Serotonin is neurotransmitter derived from the amino acid tryptophan found in the gastrointestinal tract regulating intestinal movements.
- Serotonin is also stored by platelets in the blood stream and participates in hemostasis serving as vaconstrictor.
- In the CNS serotonin regulates aggression, anxiety, appetite, cognition, learning, memory, mood, nausea, sleep, and thermoregulation.
- Amino acid neurotransmitters are classified into two main groups, inhibitory amino acids (IAA) and excitatory amino acids (EAA).
- IAA are depressors of the post-synaptic cells and include GABA, glycine, β-alanine, and taurine.
- EAA include L-glutamate, L-aspartate, L-cysteine, and L-homocysteine.
- Neuropeptides include substance P and enkephalins.
- Substance P is a neuropeptide of the tachykinin family of peptides.
- Substance P functions as a neurotransmitter as well as a neuromodulator.
- Substance P is released from the terminals of sensory nerves of the brain and spinal cord and is associated with inflammatory processes and pain.
- Enkephalins are endogenous ligands of the delta-opioid (δ-opioid) receptor.
- Activation of δ-opioid receptors produces some analgesia.
- Gasotransmitters are small molecules that freely cross membranes, do not require a cognate receptor to mediate their effects, and can function as endocrine, paracrine, and autocrine regulators.
- Gasotransmitters in the animal body include NO, H2S and CO.
- NO is also known as the “endothelium-derived relaxing factor”, a powerful vasodilator with a short half-life.
- H2S is a signalling gas produced from the amino acid cysteine.
- H2S acts as a relaxant of smooth muscle and as a vasodilator.
- CO is a signalling molecule in the neuronal system, involved in the regulation of neurotransmitters and neuropeptide release, learning and memory, response to odour, adaptation, modulation of inflammatory responses and muscle relaxation.
6.5 Glossary
5-HT – 5-hydroxytryptamine, or serotonin
ACh – acetylcholine
ACPD – 1-amino-1,3-dicarboxycyclopentane a chemical compound that binds and activates the mGluRv with no effect on the iGluR.
AMPA – 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid
ADHD – attention deficit hyperactivity disorder
AS – autonomic nervous system
AV – atrioventricular
cAMP – 3′-5′-cyclic adenosine monophosphate
cGMP – 3′-5′-cyclic guanosine monophosphate
CNS – central nervous system
CO – carbon monoxide
EAA – excitatory amino acids
GABA – γ-aminobutyric acid
GI – gastrointestinal tract
Gi – inhibitory G protein
Gq – phospholipase C-coupled G protein
Gs – stimulatory G protein
GPCR – G protein coupled receptor
H2S – hydrogen sulphide
IAA – inhibitory amino acids
iGluR – ionotropic glutamate receptor
mGluR – metabotropic glutamate receptor
LGIC – ligand-gated ion channels
MLCK – myosin light chain kinase
NADPH – nicotinamide adenine dinucleotide phosphate
NMJ – neuromuscular junction
NO – nitric oxide
NOS – nitric oxide synthase
PIF – prolactin-inhibiting factor, also called PIH or prolactostatin
PIH – prolactin-inhibiting hormone, identical to PIF
PNS – peripheral nervous system
RLS – restless legs syndrome
SNS – somatic nervous system
SSRI – selective serotonin re-uptake inhibitor
- Choline is a positively-charged quaternary ammonium compound that forms the headgroup of one the phospholipids of cellular membranes. Because the human body makes too little choline for complete health, choline is sometimes considered a vitamin. ↵
- Cognition is a group of mental processes that includes attention, memory, producing and understanding language, learning, reasoning, problem solving, and decision-making. ↵
- Several diseases of the nervous system are associated with dysfunctions of the dopamine system, including Parkinson’s disease, schizophrenia attention deficit hyperactivity disorder (ADHD) and restless legs syndrome (RLS). A variety of highly addictive drugs, including stimulants such as cocaine and methamphetamine act directly on the dopaminergic system. ↵
- Mast cells are a type of white blood cell derived from the immune system rich in granules with histamine and the anticoagulant heparin. They play a tissue protective role in wound healing, angiogenesis, immune tolerance, defense against pathogens, and in the blood–brain barrier. ↵
- Chemotaxis refers to a movement of cells along concentration gradients of a signal, either toward or away from the signal. ↵
- Enterochromaffin cells are a type of neuroendocrine cells located in the epithelia lining and the lumen of the digestive and respiratory tract that secrete serotonin. ↵
- Serotonin contributes to the feelings of wellbeing therefore the serotonin receptors are the target of a variety of pharmaceutical drugs, including many antidepressants and antipsychotics. Drugs termed selective serotonin re-uptake inhibitors (SSRIs) inhibit the reuptake of serotonin into the presynaptic cell, increasing the serotonin available to bind the postsynaptic receptor. Thus, SSRIs are used in the treatment of depression and anxiety disorders. ↵
- Free radicals are atoms or groups of atoms with an unpaired number of valence electrons. ↵

