10. Respiration
10.1 Introduction
10.2 Cellular Respiration
10.3 Gas Exchange
10.4 The Mammalian Respiratory System
10.5 Respiration in Other Animals
10.6 Summary
10.7 Suggested Readings
10.8 Glossary
10.1 Introduction
– Jim LeBlanc
The last thing Jim LeBlanc could recall was the tingling feeling of little bubbles on his tongue, as the moisture boiled off. It started as a normal day at work at NASA, but a hose on the space suit he was testing became disconnected. Without the atmosphere’s weight of one kilogramme per square centimetre to keep air in his lungs, the last few molecules of oxygen left his lungs in seconds, and within a few more seconds he passed out. Fortunately, technicians outside the test room were able to rescue him quickly, before the lack of oxygen could permanently damage his brain.
How long can you survive without food?[1]
Without water?[2]
Without air?[3] (See footnotes for answers).
If you consider the answers to these three questions, it’s clear that getting oxygen from the air into the body is critically important for survival. Your body gets its energy by combining nutrients with oxygen.
Why is energy stored in this way, with two separate chemical components that need to be combined shortly before use? Learn why, in the next section, on cellular or chemical respiration.
10.2 Cellular Respiration
As you learned in Chapters 1 (Introduction) and 8 (Digestion), living things need a way to store the energy they capture from sunlight.
Plants and other autotrophs accomplish this long-term energy storage by transferring electrons. They use electron transfer to create good electron donors, mostly sugars and fats, and an eager electron acceptor, almost always molecular oxygen, O2. But living things have no way to store a large amount of oxygen inside their bodies: Oxygen is a small molecule with weak electrostatic interactions, so it’s a gas at the sorts of temperatures and pressures where life is possible. And even if living things were able to store a lot of oxygen in their bodies, the oxygen would cause an intolerable amount of damage: Oxygen is an avid electron acceptor, and in grabbing electrons wherever it can, it sets off a chain of chemical reactions that damages a wide range of biological molecules.
That means there’s only one thing for an autotroph to do: Release the oxygen into the surrounding air, or release it into the surrounding water if the autotroph lives in water. And that’s how we ended up an atmosphere that’s 21% oxygen. See Figure 10.1.
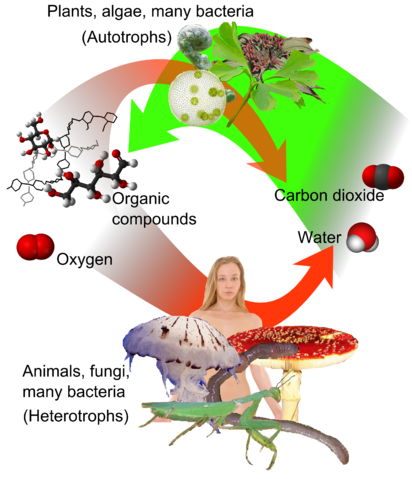
To build good electron donors, autotrophs need the two elements carbon and hydrogen. Hydrogen (Greek: húdōr, water; genḗs, born of) is easy to get, as it’s part of the water (H2O) molecule. Carbon dioxide (CO2) is the source of carbon for photosynthesis. To review two chemistry terms, reduction is the addition of electrons to an atom or molecule, while oxidation is the loss of electrons. So you can think of photosynthesis as storing the energy of sunlight in oxidised oxygen in the form of O2, plus reduced carbon and hydrogen in the form of organic compounds. [*** the way I had written this before was unreadable: “reduced carbon- and hydrogen- containing organic compounds” May need to revise further.***]Conveniently, these organic compounds can take complex forms – they are the building blocks of life. Most of the energy plants store for later use is stored in carbohydrates and fats, but animals can also extract energy from the organic molecules that form the structures and machinery of any cell, anything that can be hydrolysed into nutrients. See Chapter 8, Digestion.
How does an organism unlock this stored energy? This is an electron transfer reaction, too, using O2 as an electron acceptor for electrons from organic compounds. The cell can use the released energy in a variety of ways, but by far most of the energy goes into making ATP, the main short-term energy currency of the cell. ATP hydrolysis drives most of the processes in the cell that need energy.
Unlocking the energy stored in oxygen and nutrients is called cellular respiration (Latin: rēspīrātiō, breathing), also known as chemical respiration. The sites of chemical respiration in eukaryotic cells are organelles called mitochondria. Mitochondria are the descendants of aerobic (O2-using) bacteria that were engulfed by anaerobic archaea that together became the eukaryotic cell later on.
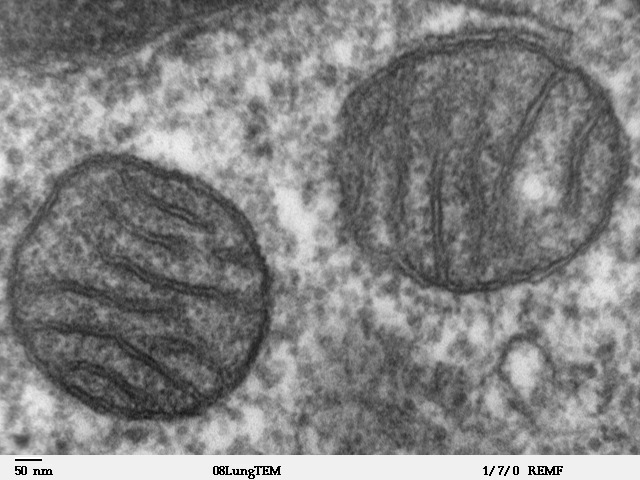 Figure 10.2 Two mitochondria inside a mammalian cell. Transmission electron micrograph. Source: Louisa Howard, Dartmouth Electron Microscope Facility, Dartmouth College, http://remf.dartmouth.edu/imagesindex.html CC 0 Public Domain, 2000.
Figure 10.2 Two mitochondria inside a mammalian cell. Transmission electron micrograph. Source: Louisa Howard, Dartmouth Electron Microscope Facility, Dartmouth College, http://remf.dartmouth.edu/imagesindex.html CC 0 Public Domain, 2000.
The end products of aerobic respiration are carbon dioxide and water.[5] For heterotrophs such as ourselves, the carbon dioxide is a waste product that would cause us problems if we let it build up in our bodies.
This means that animals need to get molecular oxygen from their environment, and at the same time get rid of excess carbon dioxide. Since both substances are gases, this process is called gas exchange. Which brings us to two older meanings of the word “respiration”: Respiration can refer to gas exchange of an organism with its environment, releasing CO2 and absorbing O2. And respiration can refer to breathing. As you can see, the three meanings of the word “respiration” are different, but related. We’ll look at gas exchange in the next section, and breathing in the section after that.
Remember how rapidly Jim LeBlanc lost consciousness? In another four or five minutes, irreversible brain damage would have happened. Can you think of reasons why the brain is especially sensitive to oxygen starvation, compared with other organs?[6] Hint: What do neurons need to maintain, despite thousands of action potentials? (See footnote for answer).
10.3 Gas Exchange
Tasks and challenges for the respiratory system A respiratory system has to accomplish two things:
- Transport oxygen in from the environment.
- Transport carbon dioxide out to the environment.
This is called gas exchange.
In most animals, there are actually three transport steps needed here:
- Gas exchange between the environment and the body fluids.
- Transport of the body fluids to and from all of the tissues of the body.
- Gas exchange between the body fluids and all of the cells of the body.
In this chapter, we discuss gas exchange with the environment in more detail than the other two tasks. The circulatory system’s jobs include distributing oxygen to all tissues of the body, and collecting and transporting carbon dioxide from all tissues of the body, so we’ll discuss those tasks in more detail in Chapter 11 (Circulation).
The respiratory system’s job is to exchange gases with the environment. This results in a challenge for any respiratory system: How to exchange gases with the environment without exchanging other materials with the environment. Can you think of things that the body needs to keep out? Can you think of things the body needs to keep in? You heard about a very similar challenge in Chapter 8 (Digestion).
Therefore, the respiratory also needs to:
- Keep out foreign objects, such as dust, silt, smoke, and most importantly, pathogenic germs.
- Avoid losing too much water to the environment.
There is one more challenge that’s important to think about: The body’s metabolic rate varies over time. Sometimes it expends a lot of energy. And sometimes it expends relatively little energy. Despite wide fluctuations in oxygen consumption and carbon dioxide production, the body needs to maintain homeostasis of the concentrations of both these chemicals. So the respiratory system needs to adjust its capacity for gas exchange accordingly. Carbon dioxide and oxygen sensors in various parts of the body need to talk with the brain, and the brain needs to talk with the respiratory system.
Properties of liquids and gases. Before discussing gas exchange in detail, it would be worth our time to review some properties of gases and liquids. Gas and liquid are two of the three phases of matter, the third phase being solid. Unlike solids, both gases and liquids are fluids. In other words, they can flow. But the two fluid phases are different from each other in important ways. In a liquid, the molecules stick to each other due to electrostatic forces. Water molecules stick to each other particularly strongly through hydrogen bonds. Each water molecule can form up to four hydrogen bonds at a time, and at room temperature, each water molecule breaks and forms hydrogen bonds billions of times a second, hydrogen bonding with an average of just over three neighbours at a time. Hydrogen bonds are so strong that the leaves of a 100 m tall tree can pull a chain of water molecules all the way from the roots. This property of water is called cohesion, and is one reason why your lungs don’t normally separate from your chest wall no matter how hard you breathe, even though the only connection is through the body fluid in the thoracic cavity.
Another property of liquids is surface tension, a force that shrinks the boundary between a liquid and another fluid that can’t mix with it. Water shows strong surface tension with air and oils, because water molecules at the interface have fewer opportunities for hydrogen bonding than water molecules in the interior. As you saw in Chapter 8 (Digestion), this poses a challenge for digestion of fats. Can you see how surface tension also poses challenges for gas exchange with air?
Because the molecules in a liquid are right next to each other, liquids are hard to compress. This contrasts with gas. In a gas, the molecules do not interact much with each other, other than collisions between molecules. When gas molecules hit a surface, they exert a force against that surface, and vice versa. The pressure exerted by a gas is proportional to how frequently gas molecules hit a surface. As a result, it is easy for an externally-applied force to compress a gas. For example, doubling the force per unit area shrinks the volume occupied by a gas by half (Boyle’s law), if you keep the temperature constant. In other words, the gas pressure at a given temperature is determined only by the number of gas molecules per unit volume.
So why doesn’t the entire atmosphere escape into outer space in just a few minutes?
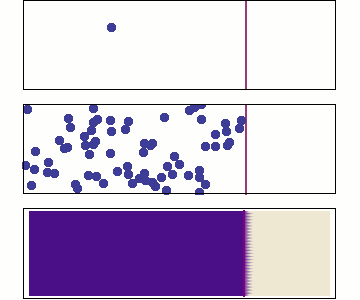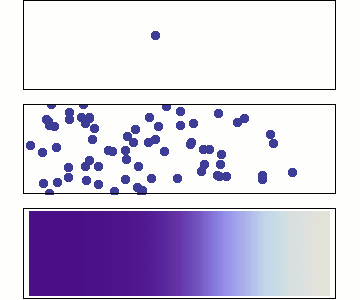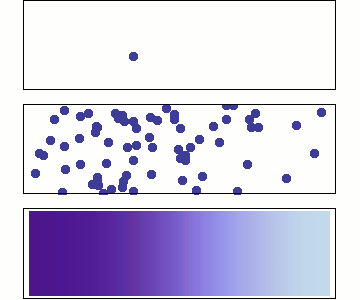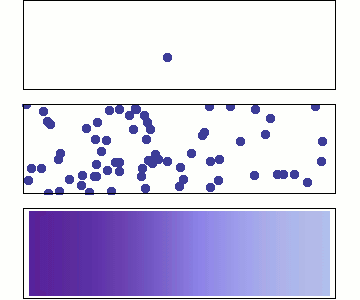
The atmosphere is held in place by the earth’s gravity. In other words, atmospheric pressure is due to the weight of the air. At sea level, the weight of the air above ground is enough to raise a column of mercury (a very heavy liquid) by 760 mm, so we say standard air pressure is 760 mmHg. Since molecules in a gas don’t interact much with each other, a useful concept is the partial pressure of each gas in a mixture of gases. See Figure 10.3. For example, the partial pressure of oxygen in air at sea level is around 160 mmHg, and the partial pressures of all the other gases in air add up to 600 mmHg for a total air pressure of 760 mmHg.
In a gas mixture such as air, the partial pressure of each gas is a measure of what proportion of the mixture is that gas. In a liquid, the partial pressure of a dissolved gas is a measure of what the partial pressure would be in a gas in equilibrium with the liquid. For example, the partial pressure of oxygen in water that’s in equilibrium with air at sea level is 160 mmHg (Air is 21% oxygen, and standard atmospheric pressure is 780 mmHg. 21% × 760 mmHg = 160 mmHg).
Oxygen and carbon dioxide in water. Oxygen is a nonpolar molecule, and as a result, it has only weak electrostatic attractions with other molecules. This has important consequences. For instance, oxygen dissolves poorly in water because oxygen molecules tend to be excluded by the strong hydrogen bonds between water molecules. The amount of oxygen that can be dissolved in water at atmospheric pressure is far less than there is in an equivalent volume of air at the same pressure.
If oxygen doesn’t dissolve well in water, how do animals deliver oxygen to their tissues? The answer is that animals have proteins that reversibly bind to oxygen. In many animals, a specialized oxygen-binding protein called haemoglobin carries oxygen inside red blood cells. Haemoglobin increases the oxygen carrying capacity of blood seventy fold compared with water.
How long would your body’s oxygen supply last if you can’t breathe, and why?
Let’s do a few quick calculations. Let’s start with the amount of energy your body needs: A young adult in Canada eats an average of 2500 kcal[7] of food each day. But those 2500 kcal include the energy needed for physical activity, plus food eaten far in excess of your body’s needs. At a comfortable temperature at rest, an adult human body needs only about 1500 kcal of food a day. That works out to roughly 70 Watts[8], the same as a relatively dim, old-style incandescent light bulb. The amount of energy the body gets from a given amount of oxygen doesn’t vary much between foods, so 70 Watts corresponds to 5 milligrammes O2 per second, or roughly one pound O2 per day, regardless of diet.
Now, let’s take a look at how much oxygen is stored in the body. The average adult human body contains five litres of blood (a little over a gallon), containing three quarters of a kilogramme of haemoglobin (a little over one and a half pounds). In a healthy human at sea level, the haemoglobin in oxygenated blood is roughly 96% saturated with oxygen, and around 70% saturated in deoxygenated blood. This works out to be a total of around 1.2 grammes O2 attached to haemoglobin in a typical adult human. Based on the energy usage we discussed in the previous paragraph, that’s a 4 minute supply. By contrast, five litres of pure water can dissolve only about 0.025 grammes of oxygen at equilibrium with air. Of course, the tissues of the rest of the body also contain dissolved oxygen, but it’s still only a small amount compared with the oxygen carried by haemoglobin in the blood. So how do some people manage to hold their breath for ten minutes? Which pool of oxygen have we missed? The air in the lungs! If you breathe in as deeply as you can, your fully inflated lungs will contain roughly 6 L of air, most of it in the alveoli where gas exchange takes place. How much oxygen does 6 L of air contain? Warm, moist air is around 20% O2, so that’s 1.2 L of oxygen. You might remember from Chemistry class that one mole of gas at 37°C at sea level occupies (V = nRT/p) 25.4 L. Knowing that the molecular mass of O2 is 16 × 2 = 32 g/mol, this works out to be 32 g/mol × 1.2 L / 25.4 L/mol = 1.5 grammes of oxygen. So, a full pair of lungs contain roughly a 5 minute supply of oxygen. With a 4 minute supply of O2 in a normal adult’s blood and a 5 minute supply in the lungs, the world record for holding one’s breath – around 10 minutes – sounds incredible! Especially given that involuntary control of breathing normally overwhelms voluntary control with only a small rise in blood carbon dioxide levels, and given that most people would lose consciousness by the time their stored oxygen drop below a third of normal.
Carbon dioxide also dissolves poorly in water, but the carbon dioxide carrying ability of water is greatly increased by the fact that carbon dioxide can react reversibly with water to form bicarbonate, as follows:
CO2 + H2O ↔ HCO2– + H+
Notice the double-headed arrow, meant to indicate that the reaction goes in both directions in the body.
Also notice that a hydrogen ion is released, and remember that pH is a measure of the hydrogen ion concentration. When carbon dioxide reacts with water, it makes the water more acidic by creating bicarbonate, an acid. This reaction is slow in pure water, but the enzyme carbonic anhydrase speeds it, and the reverse reaction, approximately one million fold. Carbonic anydrase is abundant throughout the animal body.
Diffusion and flow. There’s one more concept that’s important to understand, in order to understand gas exchange and gas transport in biological systems. At room temperature in water that isn’t flowing, a dissolved oxygen molecule moves at four metres per second on average. But the same oxygen molecule takes an average of four minutes to get one millimetre from its starting point. Why is this the case? Think it over for a moment, if the answer isn’t obvious to you.
The answer is that the oxygen molecule doesn’t take a straight path – it collides with other molecules, changing direction each time, such that it takes a random walk. See Figure 10.4. This random walk is called diffusion. The same applies to oxygen molecules in air, only the average speed of oxygen molecules is a hundred times faster, 400 metres per second, so the speed of diffusion is a hundred times faster in air than in water. Either in air or in water, diffusion is very fast over small distances, and very slow over longer distances.
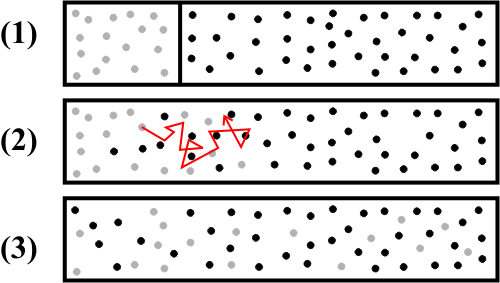
Not all animals have respiratory systems. If an animal is tiny, barely large enough for us to see, or if it has a very slow metabolic rate, or if all of its cells are close to the fluid that surrounds the animal, it may not need a respiratory system. For example, water from the outside environment flows past most of the cells of a sponge, propelled by the flagella of the choanocytes (See Chapter 1). But most animals do, in order to overcome the limitations of gas transport by diffusion.
Most animal bodies are large enough that transport by bulk flow is much more efficient than diffusion, for transport between distant parts of the body. For example, blood typically flows at one millimetre per second in capillary vessels, the smallest blood vessels in the body. And the velocity of blood in large vessels can be hundreds of times as fast as in capillaries. Remember how long it takes for an oxygen molecule to cross one millimetre at room temperature? At body temperature, it takes three minutes by diffusion, faster than at room temperature, but not by much. A metabolically active animal that’s more than a fraction of a millimetre across is going to have problems if it relies on diffusion alone.
Consequently, most animals have one or more kinds of circulatory pumps to drive the flow of liquids inside their bodies. Chaper 11 (Circulation) covers that topic.
Since oxygen molecules are nonpolar and small, cell membranes pose no barrier to the diffusion of oxygen. But of course, cell membranes pose a barrier to bulk flow of oxygen. By “bulk flow”, we mean flow of the fluid the oxygen is carried in.
The amount of passive diffusion across a barrier to bulk flow is going to be directly related to the number of collisions of solute molecules with that barrier. A molecule colliding with the barrier will have an equal chance of going back or going across, independently of other solute molecules. As a result, the rate of diffusion across a barrier is directly proportional to solute concentration on one side. See Figure 10.5.
Of course, some of the same solute will be already on the other side of the barrier. The rate of diffusion back will be proportional to the concentration on that side. So we have two rates of diffusion, one in each direction. So the net rate of diffusion, the net transfer of solute across the barrier, will be proportional to the difference in concentrations between the two sides. Notice that this means that net transfer of solute by passive diffusion will always be down a concentration gradient, from the region of greater concentration to the region of lower concentration.
To recap, if all other factors are equal, doubling the difference in concentration will double the net rate of passive diffusion across the barrier. So far, we’ve been discussing diffusion through an imaginary plane or 2-dimensional surface, but similar principles apply to diffusion through a 3-dimensional volume of non-flowing material. The steeper the concentration gradient, the faster the net movement of solute will be. See Figure 10.5.
Remember that transport over longer distances tends to be much slower by diffusion than by bulk flow. So to maximise the rate of transport, barriers need to be as thin as possible. You can understand this in terms of concentration gradients: If the barrier is thin, the concentration gradient will be steep, resulting in fast transfer of molecules across the barrier by diffusion; If the barrier is thick, the concentration gradient will be shallow, resulting in slow transfer of molecules. If all other factors are equal, halving the thickness of the barrier will double the net rate of passive diffusion across the barrier.
There’s a second way to increase the rate of passive diffusion across a barrier. Remember, the amount of diffusion across a barrier is directly proportional to the number of solute molecule collisions with that barrier. Therefore, the rate of diffusion will be directly proportional to the area of that barrier. If all other factors are equal, doubling the surface area doubles the rate of passive diffusion across the barrier.
The effect of surface area becomes important when you consider animal bodies of different sizes. Suppose we keep the shape of an animal’s body constant, and vary the size: What happens if we double the length, width, and height of the animal? The body’s volume would increase 2 × 2 × 2 = 8 fold, such that the amount of living tissue that needs to communicate with the outside environment increses 8 fold. But the body’s surface area would only increase 4 fold. So the gas exchange needs of each cell in the body – the need to get oxygen and get rid of carbon dioxide – would be served by only 4/8 = 1/2 as much surface area (see Figure 10.6). Clearly, there’s a limit to how much the size of an animal’s body can increase without having to develop special adaptations to increase the respiratory surface area. We’ll return to this topic later in this chapter when we discuss the anatomy of the lung.
 Figure 10.6 Respiratory systems are all about bringing a large as practical surface area of blood close to fluid from the outside environment. In this photo, the axolotl’s exernal gills are pink with blood. Gas exchange with the surrounding water happens through the large surface area of the gills. Source: Henry Mühlpfordt, https://commons.wikimedia.org/wiki/File:Leucistic_Axolotl_front_2010-02-24.JPG CC BY-SA 3.0, 2010.
Figure 10.6 Respiratory systems are all about bringing a large as practical surface area of blood close to fluid from the outside environment. In this photo, the axolotl’s exernal gills are pink with blood. Gas exchange with the surrounding water happens through the large surface area of the gills. Source: Henry Mühlpfordt, https://commons.wikimedia.org/wiki/File:Leucistic_Axolotl_front_2010-02-24.JPG CC BY-SA 3.0, 2010.
As mentioned before, the rate of diffusion across a barrier depends on the barrier’s thickness. One factor that can increase a barrier’s effective thickness is an unmixed layer of fluid on the outside. Most fluids, such as air and water, have a property called viscosity. Viscosity is the fluid’s mechanical resistance to shear, and results in mechanical resistance to flow past a surface. As a result, the fluid directly next to the surface doesn’t flow at all. This lack of flow next to a surface suppresses mixing, unless the fluid is actively stirred. The unstirred layer of outside water or air that limits gas exchange by increasing the distance that gases have to diffuse through. To mix the air or water to make this layer as thin as possible, animals usually need a respiratory pump in addition to their circulatory pumps.
Figure 10.7 Breathing in an alligator. The muscles and structures involved are quite different from in a mammal, nevertheless breathing looks quite similar. Source: Claessens, O’Connor, and Unwin (2009) “Respiratory Evolution Facilitated the Origin of Pterosaur Flight and Aerial Gigantism”. PLOS ONE. DOI:10.1371/journal.pone.0004497. PMID 19223979. PMC: 2637988 CC BY 4.0.
The best way to understand the idea of a respiratory pump is to look at a real-life example: Our lungs. The next section of this chapter discusses the respiratory system in mammals.
10.4 The Mammalian Respiratory System
10.4.1 Anatomy
10.4.2 Control of Breathing
10.4.3 What Could Possibly Go Wrong?
10.4.4 Secondary Functions
Anatomy
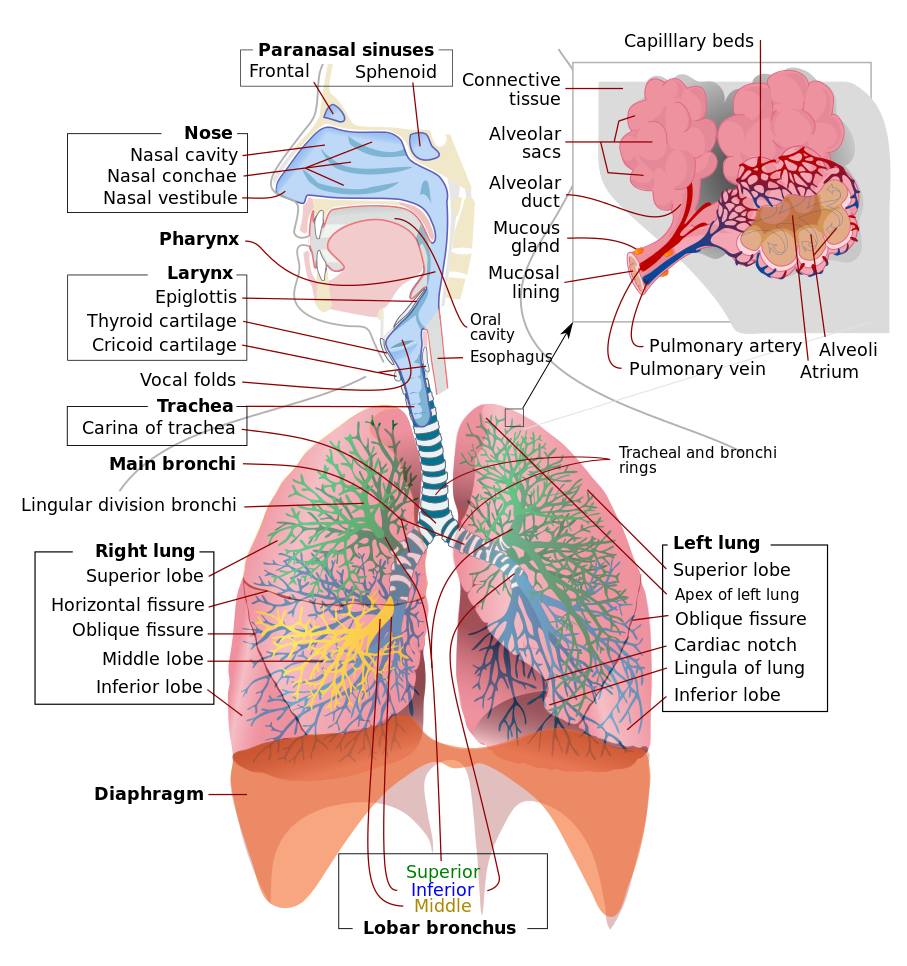 Figure 10.8 Respiratory system of a mammal. Source: Mariana Ruiz Villarreal / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Respiratory_system_complete_en.svg Public Domain, 2007.
Figure 10.8 Respiratory system of a mammal. Source: Mariana Ruiz Villarreal / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Respiratory_system_complete_en.svg Public Domain, 2007.
All mammals breathe air. This requires a large surface area where deoxygenated blood comes close to air, so that the blood can pick up oxygen and release carbon dioxide by diffusion. This blood-air interface is in a pair of spongy, elastic sacs called the lungs. A set of pipes – the bronchi, trachea, and larynx – connect the lungs to one or more openings to the outside air – the nose and mouth. See Figure 10.9 for an overview of the human respiratory system. In this section of the respiration chapter, we’ll look at some of the anatomical features of the respiratory system, starting with the nose and mouth, following the air passages all the way to the gas exchange surface in the lungs, and ending with a short description of the muscles in the torso that drive breathing.
Most mammals breathe through a pair of small openings in the face, the nostrils (Old English: nosu, nose; thirel, hole)[10] (In a medical setting, the nostrils are sometimes referred to by their Latin name, nares). Land mammals are able to breathe through their mouth in addition to their nostrils, because the respiratory (air) tract intersects with the alimentary (food) tract.
Why not breathe through the mouth all the time? After all, the mouth is a much bigger opening than the nostrils, so it poses less resistance to air flow. Indeed, during particularly heavy physical exertion, many mammals will breathe through their mouth. Nevertheless, the nose performs several functions that cannot be replaced by the mouth.
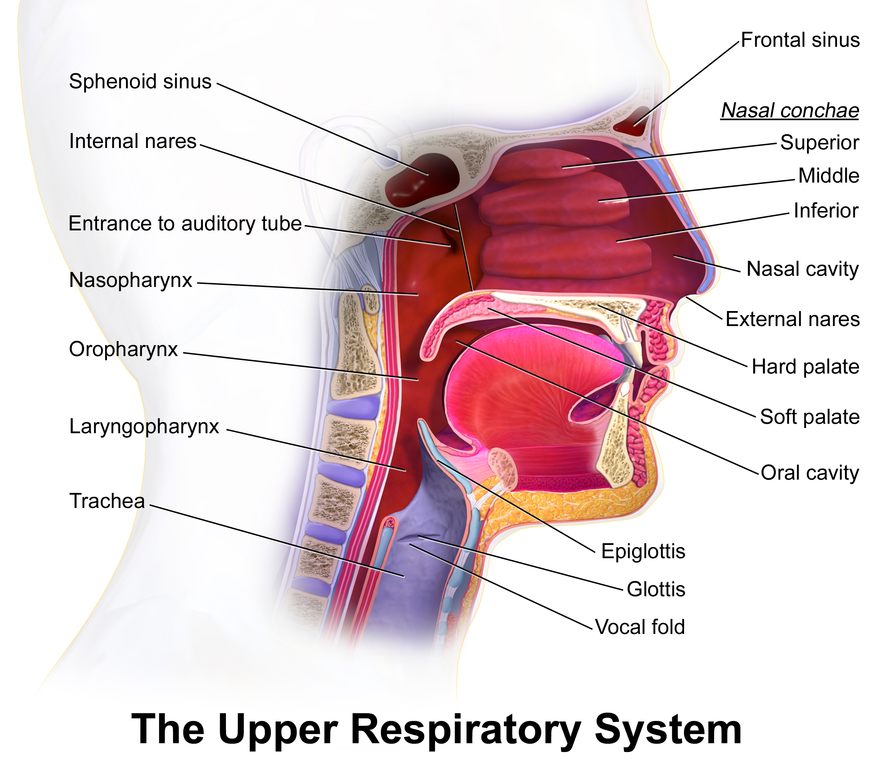 Figure 10.9 Upper respiratory anatomy in humans. Source: Bruce Blausen / Blausen.com staff, “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). ISSN 2002-4436, https://doi.org/10.15347/wjm/2014.010 CC BY 3.0, 2014.
Figure 10.9 Upper respiratory anatomy in humans. Source: Bruce Blausen / Blausen.com staff, “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). ISSN 2002-4436, https://doi.org/10.15347/wjm/2014.010 CC BY 3.0, 2014.
In most mammals, the nostrils lead to two chambers that contain elaborate, scroll-like structures called respiratory turbinates, also known as the conchae (from Latin: concha, from Greek konche, seashell). The turbinates are rigid sheets of tissue backed by bone or cartilage. See Figure 10.10. In cross-section, a turbinate resembles a conch shell, so turbinates are also known as conchae (from Latin: concha, from Greek konche, seashell). This shape maximises the surface area of mucosa that the inhaled and exhaled air passes over.
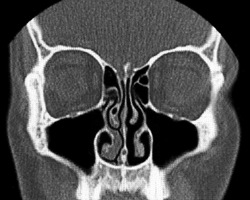 Figure 10.10 Cross-section of a human face, showing the turbinates in the nasal cavities. Source: Simplicius / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Concha_nasalis.gif CC BY-SA 3.0, 2011.
Figure 10.10 Cross-section of a human face, showing the turbinates in the nasal cavities. Source: Simplicius / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Concha_nasalis.gif CC BY-SA 3.0, 2011.
The surfaces of the airways perform three important functions:
- Moisten inhaled air, and dry exhaled air
- Warm inhaled air, and cool exhaled air
- Remove dust and other particulates
The turbinates of the nose increase the surface area of the airway closest to where air enters and leaves the body.
When air is inhaled through the nose, water evaporates from the mucus covering the turbinates, cooling them and humidifying the air. When air is exhaled through the nose, the moisture condenses on the turbinates. In this way, the nose helps to cut the loss of water from the body by more than half. Conserving water is important for the survival of most land animals. A membrane that’s thin and permeable enough to allow rapid gas exchange will also allow the exchange of water molecules, so in on order to reduce loss of water from the gas exchange surfaces of the lungs, moisture has to be recovered from the air being breathed out.
Likewise, the turbinates warm inhaled air, and cool exhaled air, helping to conserve heat in the body. Conversely, in hot weather, some animals cool themselves by panting through the mouth, if they have a limited ability to sweat. By breathing through the mouth, the animal can cool itself both by evaporation of water from the airways and lungs, and by the release of warmed air.
A third function of the turbinates is to trap dust, spores, and other particles so that they cannot lodge in the lungs. Since airborne pathogens – viruses, bacteria, and fungal spores – often ride on larger particles, the layer of mucus on the turbinates serves as a first line of defence against infection. Particles stick to the mucus, and the mucus is continuously transported, like a conveyor belt by the beating of the cilia of the mucosal cells.
Even though we’ve been discussing these three functions in reference to the turbinates, all of the air-conducting passages of the respiratory system also secrete mucus and are lined with ciliated cells. All of the air-conducting passages moisten and warm inhaled air, and trap and transport particulates. If the airways dry out, the cilia would be immobilised, and delicate cells of the epithelia would also suffer damage.
A distinctive smell can instantly transport you back many years to your grandparents’ house. The aroma of food can perk up your appetite. Traces of MHC (Major Histocompatibility Complex) proteins, a smell you aren’t even consciously aware of, helps determine who you’ll fall in love with and marry.
Chemicals present in trace amounts in the air are a rich and valuable source of sensory information for animals. The ability to detect chemical compounds in the air is called the sense of smell, or olfaction.
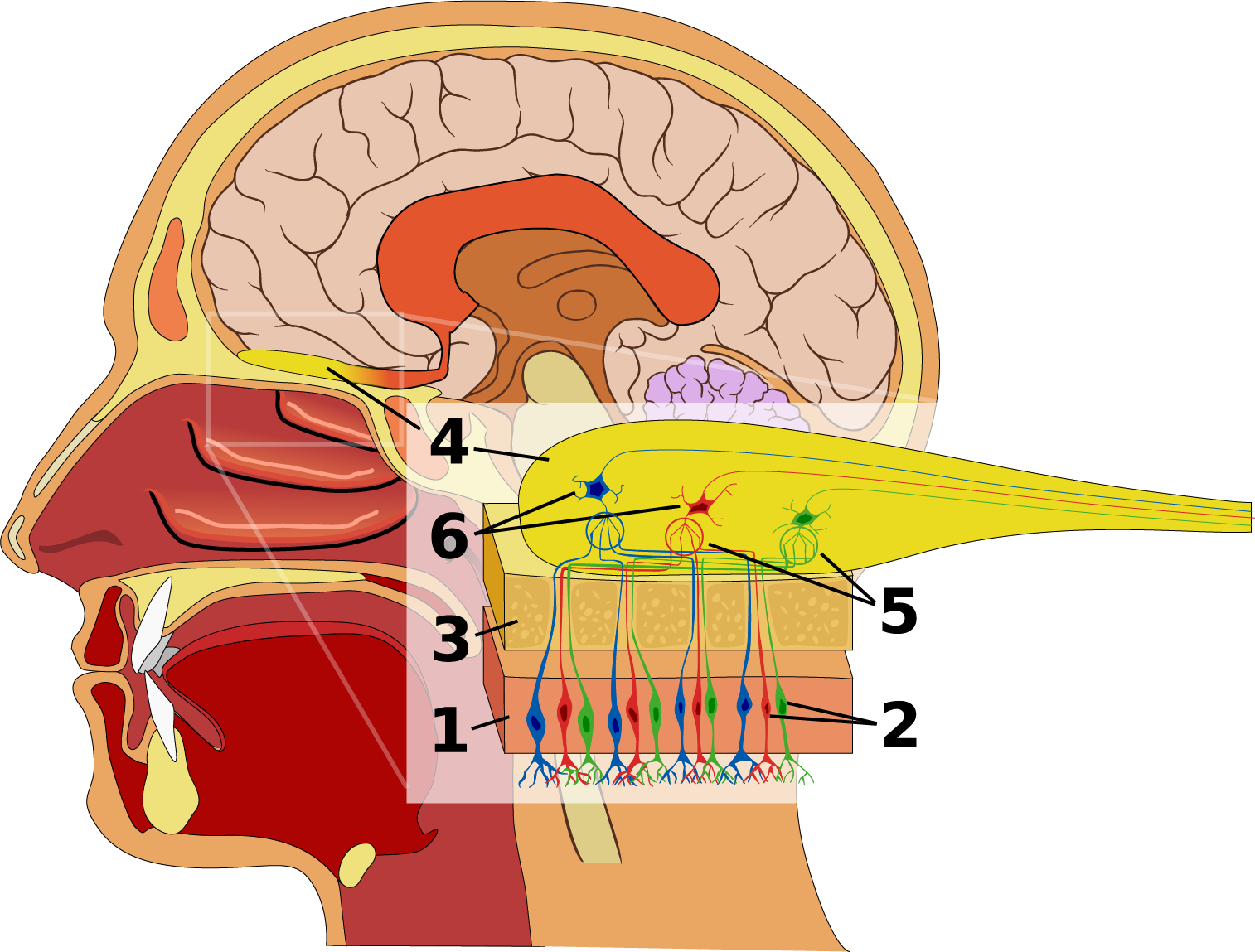 Figure 10.11 Olfactory system of the human nose. The olfactory epithelium (1) is a special region of the nasal mucosa containing millions of chemosensory neurons (2), whose dendrites end in nonmotile cilia in the mucus layer. The cilia of each chemosensory neuron bear only one odorant receptor protein, out of hundreds of different odorant receptors on the olfactory epithelium. Nerves from the olfactory epithelium lead through the bone (3) of the skull to the olfactory bulb (4), the lobe of the brain closest to the nose. In the olfactory bulb, the axons of chemosensory neurons terminate in a ball-shaped regions called a glomeruli (5), where they contact dendrites of mitral neurons and tufted neurons (6). Each neuron in the olfactory bulb collects signals from thousands of chemosensory neurons expressing the same odorant receptor protein. Source: Ignacio Icke / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Olfactory_system.svg CC BY-SA 2.5, 2007.
Figure 10.11 Olfactory system of the human nose. The olfactory epithelium (1) is a special region of the nasal mucosa containing millions of chemosensory neurons (2), whose dendrites end in nonmotile cilia in the mucus layer. The cilia of each chemosensory neuron bear only one odorant receptor protein, out of hundreds of different odorant receptors on the olfactory epithelium. Nerves from the olfactory epithelium lead through the bone (3) of the skull to the olfactory bulb (4), the lobe of the brain closest to the nose. In the olfactory bulb, the axons of chemosensory neurons terminate in a ball-shaped regions called a glomeruli (5), where they contact dendrites of mitral neurons and tufted neurons (6). Each neuron in the olfactory bulb collects signals from thousands of chemosensory neurons expressing the same odorant receptor protein. Source: Ignacio Icke / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Olfactory_system.svg CC BY-SA 2.5, 2007.
In each nasal cavity, above the respiratory turbinates, is a mucosal epithelium that’s packed with chemosensory neurons, millions in the case of humans. In many animals that have a keen sense of smell, this chemosensory epithelium is on a separate olfactory turbinate. The mucus covering the epithelium dissolves chemicals from the air. Each chemosensory neuron has several non-moving cilia that extend from its dendrite into the mucus. The cilia of each neuron have one of hundreds of different chemoreceptor proteins in the seven-transmembrane domain G-protein-coupled receptor (GPCR) family. Each chemosensory neuron bears only one of hundreds of different chemoreceptor proteins. Some odor receptors detect only one chemical compound, but most detect a range of chemicals wth similar structures. Having a few hundred different odor receptor GCPRs, each in a different set of sensory neurons, gives the human nose the ability to distinguish hundreds of chemicals from each other. Based on the pattern of which chemosensory neurons are producing action potentials and which are not, we have the ability to tell apart thousands, if not millions, of complex odors that consist of mixtures of chemicals.
At the back of each nasal cavity, there’s an opening into the pharynx, which also connects with the mouth cavity through the throat. Lower down, the pharynx splits, leading to the rest of the respiratory system through an opening called the glottis, and also to the rest of the alimentary tract through the upper sphincter of the eosophagus. See Figure 10.12. Cilia on the mucosa of the pharynx sweep mucus from nasal cavities the down to where it can be swallowed into the eosophagus.
The alimentary tract and respiratory system joining together creates a potential problem: What prevents food and drink from being breathed into the airway through the glottis? The simple answer is that the swallowing reflex closes the top of the airway, while moving the glottis out of the way. The details are too complicated to describe here, but you can get a general idea if you review the arrangement of the glottis and the voicebox, also known as the larynx, in Figure 10.12. During swallowing, various muscles move the tongue back, raise the larynx and move it forwards, and raise the upper eosophageal sphincter. These motions swing a flap of cartilage called the epiglottis over the glottis, closing it off. At the same time, a pair of elastic flaps of tissue called the vocal cords close off the airway just below the glottis, in a rigid chamber called the larynx.
Figure 10.12 Swallowing pineapple juice. When swallowed, the juice enters the lower pharynx, a passageway that the respiratory system and the alimentary tract share. Breathing has to stop, and the airway has to be temporarily closed off at the glottis. Don’t laugh, or juice will come out your nose! Source: Martin Uecker, Biomedizinische NMR Forschungs GmbH, http://www.biomednmr.mpg.de CC BY-SA 3.0
The larynx is also known as the “voice box”. If the vocal cords are tensed while air is exhaled past them, they vibrate, making a sound. In most land vertebrates, the larynx is the main site of sound production for communication between individuals.
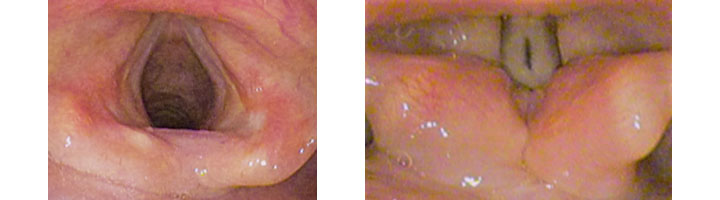
What makes the vocal cords vibrate? When the vocal cords gently close together, they block the flow of air, such that air pressure builds up behind them. When the air pressure gets high enough, the air forces the vocal cords apart. Once there is a small gap between the vocal cords, the difference in pressure creates a rapid jet of air into the larynx. With the rush of air through the gap, the air pressure between the vocal cords drops, allowing the vocal cords to snap shut, starting the cycle over again. Thus, the vocal cords chop the flow of exhaled air into many puffs per second. All of the energy to produce sound from the vocal cords comes from the flow of air from the lungs. The larynx, throat, sinusus, nose, and mouth shape and filter these pressure waves into the sound others can hear. Parts of the upper respiratory tract produce sounds as well, but the vocal cords are most often the main source of sound.
During normal breathing, the cartilage and muscles of the larynx keep the vocal cords wide apart, allowing the free flow of air.
The larynx connects to a stiff tube called the trachea, which branches into two tubes called the primary bronchi, one bronchus for each of the two lungs. Rings of cartilage support the walls of the trachea and bronchi. As you will see later in this chapter, the air pressure inside the airways is less than the outside pressure at times during the breathing cycle. Having a rigid support prevents the airways from collapsing, which would block air flow when it is needed the most during breathing.
Even though each lung has over 1000 kilometres of airways, the distance air has to travel is only a fraction of a metre. This is achieved by repeated branching. The primary bronchi branch further into secondary bronchi, which, in turn, branch many times into smaller and smaller airways. The smallest tubes are called bronchioles. In total, there are more than twenty generations of airway branching in an adult human’s lungs, such that a single airway, the trachea, leads to millions of bronchioles.
The mucosal surface of these hundreds of kilometres of airways plays an important role in protecting the lungs from harmful substances and germs in the air. Like the lining of the airways of the nose and pharynx, the lining of the larynx, trachea, bronchi, and bronchioles secrete a layer of mucus. Particles stick to the mucus, and the beating of the cilia of the mucosal cells mucus is continuously transports this mucus like a conveyor belt towards the pharynx, to be swallowed into the digestive tract.
 Figure 10.14 Epithelium of a bronchiole, showing both cells with cilia and cells without cilia. The cilia beat, driving a flow of mucus towards the pharynx, where the mucus is swallowed. Scanning electron micrograph. Source: Charles Daghlian, Dartmouth Electron Microscope Facility, Dartmouth College, http://remf.dartmouth.edu/imagesindex.html CC 0 Public Domain, 2004.
Figure 10.14 Epithelium of a bronchiole, showing both cells with cilia and cells without cilia. The cilia beat, driving a flow of mucus towards the pharynx, where the mucus is swallowed. Scanning electron micrograph. Source: Charles Daghlian, Dartmouth Electron Microscope Facility, Dartmouth College, http://remf.dartmouth.edu/imagesindex.html CC 0 Public Domain, 2004.
The width of of the airways is normally close to the optimum to make sure there is as little resistance to airflow as possible. For moderate flow rates, the viscous resistance to flow through a cylindrical tube is inversely proportional to the fourth power of the tube’s diameter. So, for example, doubling the diameter of a tube reduces the resistance to flow to one-sixteenth. In other words, for a given difference in fluid pressure between the two ends of the tube, doubling the tube’s diameter increases the flow rate sixteen-fold! A small difference in diameter can make a big difference in flow rate. The relationship between the width of a pipe and flow is especially important in the circulatory system, discussed in Chapter 11.
In the smaller airways, the cartilage rings are more often C-shaped, than complete rings. This allows smooth muscle in the airway walls to make adjustments to airway diameter. When there is a greater need for airflow, the smooth muscle relaxes, making the airways slightly wider. When the mucosa detects irritants such as dust and smoke, the smooth muscle contracts, making the airways slightly narrower. Narrowing the airways increases the opportunity for mucus to trap and remove harmful substances and particles from the air.
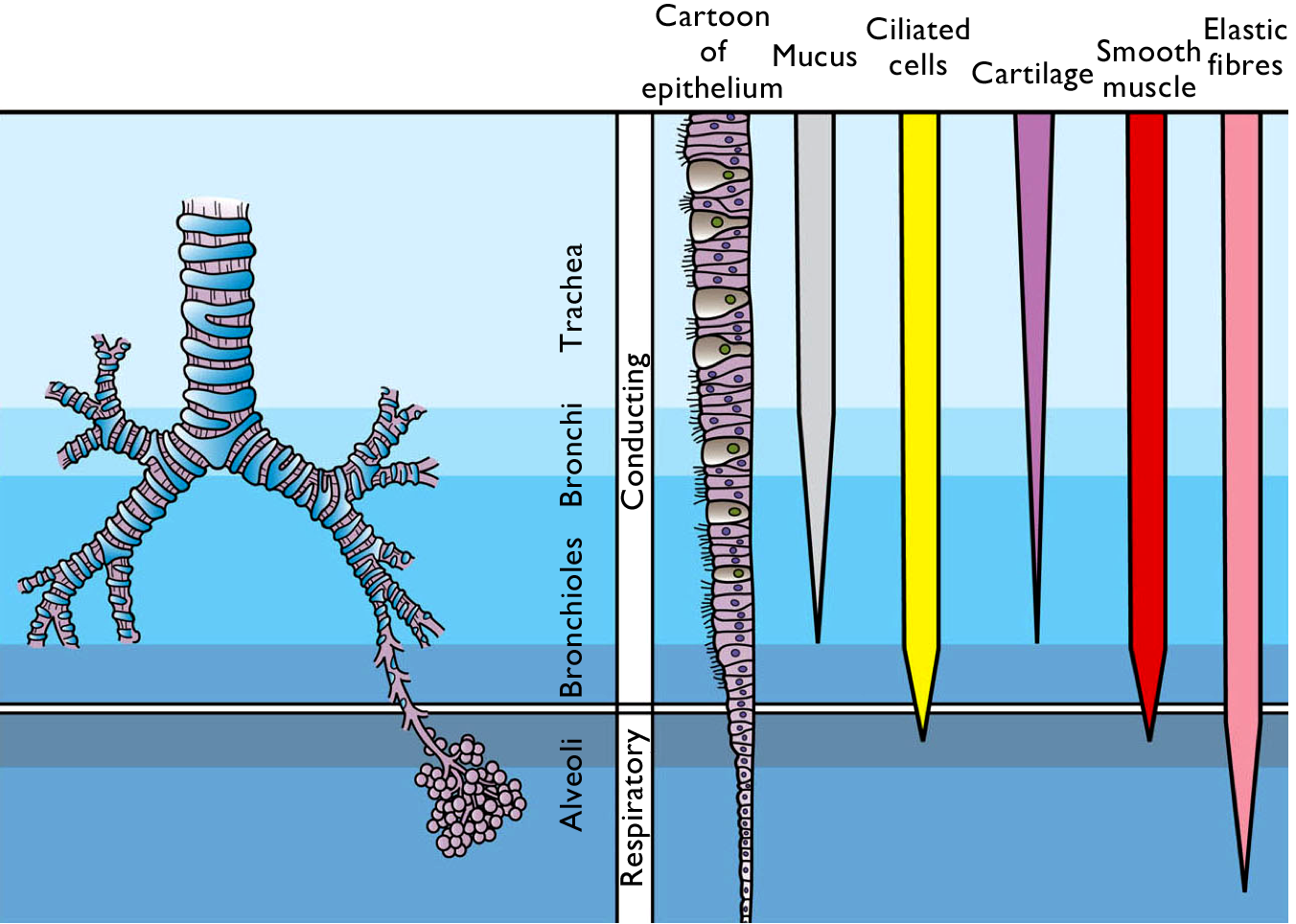 Figure 10.15 Comparison of airways in the lower respiratory tract. Source: adapted from Holly Fischer / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Respiratory_Tract_Histological_Differences.png CC BY 3.0, 2013.
Figure 10.15 Comparison of airways in the lower respiratory tract. Source: adapted from Holly Fischer / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Respiratory_Tract_Histological_Differences.png CC BY 3.0, 2013.
Once the air has been warmed, humidified, and cleaned, it is ready to come into close contact with the blood, so that the blood can pick up oxygen and release carbon dioxide.
Imagine you have a small cup of ink, roughly the volume of a standard double espresso. Where would it dry out faster: in the cup, or evenly spread out over the area of a badminton court? The total volume of blood in an adult human’s lungs is around 70 mL, slightly more than a double espresso, and the respiratory surface area, the area for gas exchange, is around 75 square metres, the area of a singles badminton court.
Most of the respiratory surface area for gas exchange in the lungs is in air-filled sacs called alveoli (Latin: alveolī, little hollows), 300 million to 800 million per lung.
In order to achieve nearly instant diffusion of gases between the air and the blood, the unstirred layer of liquid has to be as thin as possible. This means epithelial cells that are as thin as possible. The distance from air to blood in the alveoli ranges from 200 nanometres to 2 micrometres thickness, 600 nm being typical. Look at how close the red blood cells are to the air in Figures 10.16 and 10.17. It also means the layer of water on the epithelial cells needs to be as thin as possible – no room for mucus, and certainly no room for cilia! So how do alveoli stay clean? Amoeba-like cells called macrophages do what they can to engulf bacteria, dust, and debris. You can see a macrophage in the centre of Figure 10.17.
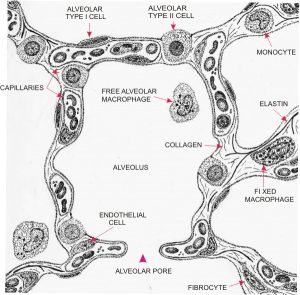 Figure 10.16 Diagram of a cross-section of an alveolus of a human lung. Source: Yves Clermont, Michael Lalli, and Zsuzsanna Bencsath-Makkai, http://audilab.bmed.mcgill.ca/HA/html/resp_21_E.html CC BY-NC-ND, 2013.
Figure 10.16 Diagram of a cross-section of an alveolus of a human lung. Source: Yves Clermont, Michael Lalli, and Zsuzsanna Bencsath-Makkai, http://audilab.bmed.mcgill.ca/HA/html/resp_21_E.html CC BY-NC-ND, 2013.
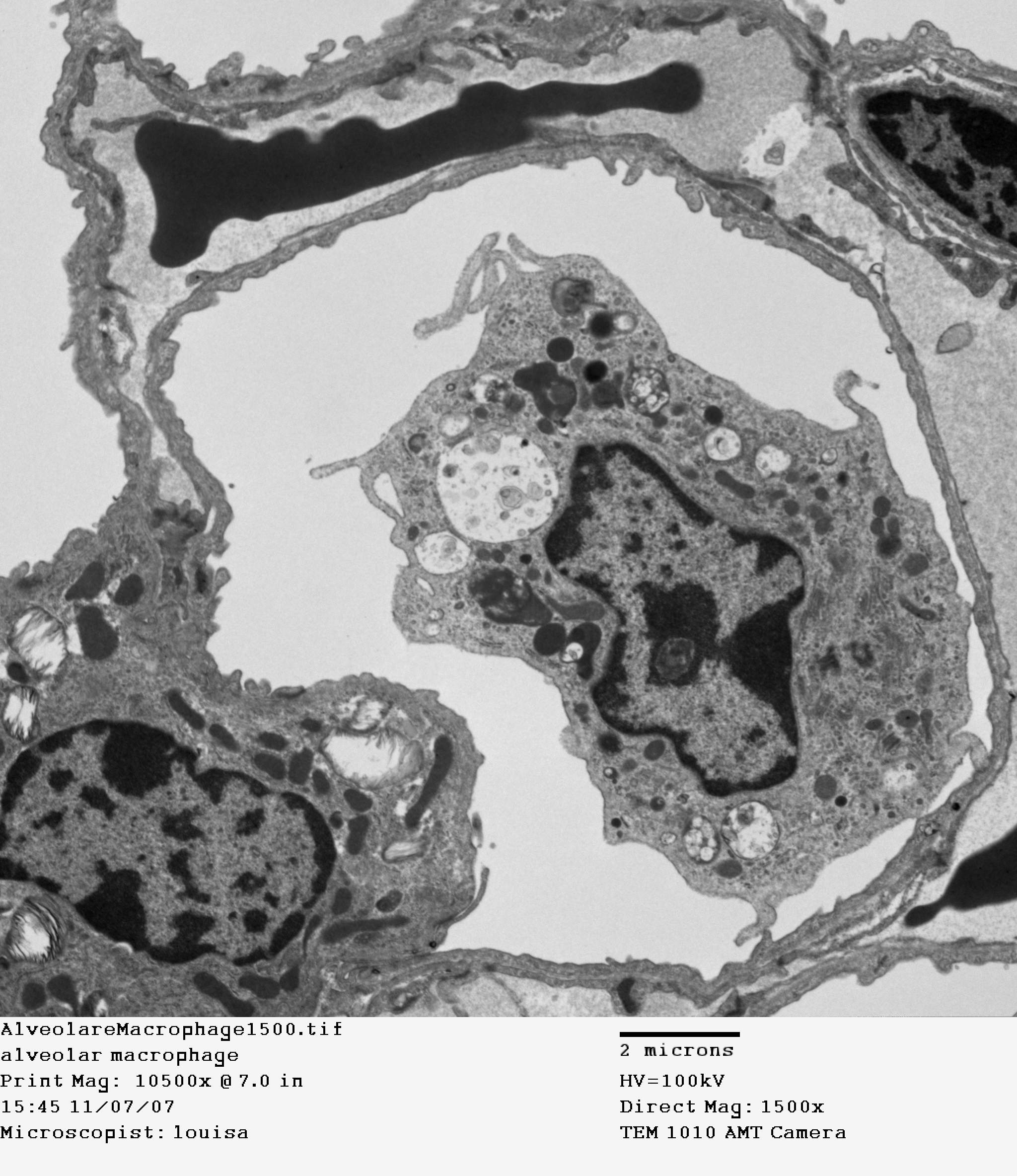 Figure 10.17 Cross-section of an alveolus. Transmission electron micrograph. Source: Louisa Howard, Dartmouth Electron Microscope Facility, Dartmouth College, http://remf.dartmouth.edu/imagesindex.html CC 0 Public Domain, 2004.
Figure 10.17 Cross-section of an alveolus. Transmission electron micrograph. Source: Louisa Howard, Dartmouth Electron Microscope Facility, Dartmouth College, http://remf.dartmouth.edu/imagesindex.html CC 0 Public Domain, 2004.
The extremely curved surface of water in the alveoli poses a challenge, because of surface tension. Imagine several hundred million microscopic bubbles, with a hole poked in each one. The force of surface tension tends to collapse alveoli, and also tends to fill them with water. To reduce surface tension to a manageable level, specialised epithelial cells in the alveoli secrete a complex mixture of several different phospholipids and proteins that function as surfactants. You can see one of these “Type II” alveolar cells in the bottom left of Figure 10.17 (the thin epithelial cells that are the main site of gas exchange are called “Type I” alveolar cells). Surfactant molecules are amphipathic, meaning one part of the molecule is hydrophilic (binds well to water), while the other side of the molecule is hydrophobic (binds poorly to water). The hydrophobic side faces the air, while the hydrophilic side faces the water, stabilising the air-water interface. During normal, resting breathing in healthy lungs, the surfactant cuts surface tension in the lungs to a negligible level. Even if a person dilutes the surfactant by inhaling to their full lung capacity, surface tension is still less than half that of pure water.
Lung surfactant is especially important in newborn babies, who have to clear their lungs of amniotic fluid and forcefully fill their lungs with air for the first time. Premature babies often have difficulty breathing, and inadequate lung surfactant is the most common reason why.
To accomodate the enormous changes in lung size that can happen during deep breathing, alveoli need to be extremely stretchy and elastic. The alveolus walls need to be extremely tough even though they are as thin as 200 nm when stretched out during inhalation. Take a second look at Figure 10.18, which shows typical lung air volumes during breathing.
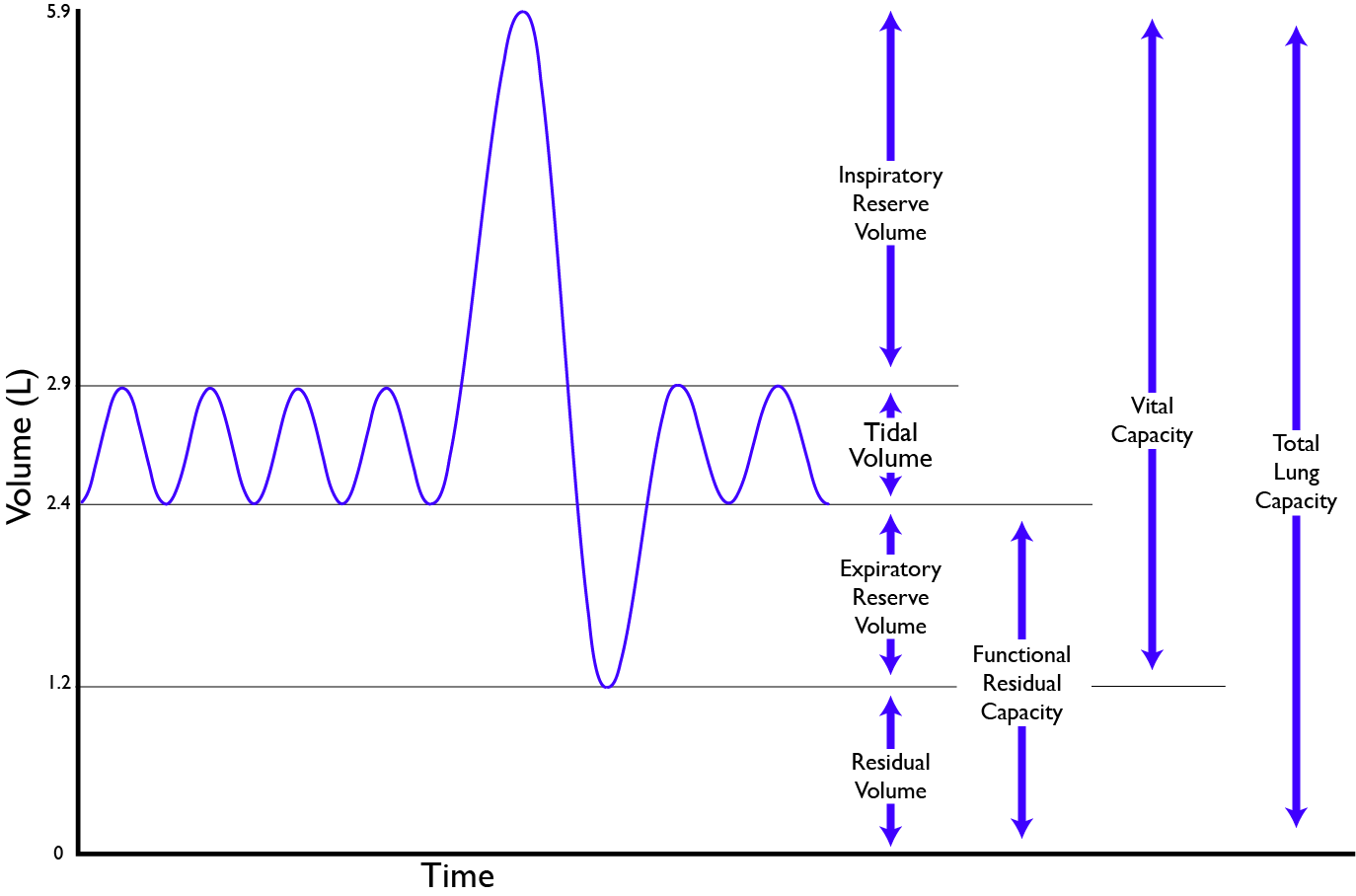 Figure 10.18 Air volumes in the lungs of a typical human adult. The tidal volume is the volume of air breathed in and out during regular breathing. CC 0 Public Domain, 2017.
Figure 10.18 Air volumes in the lungs of a typical human adult. The tidal volume is the volume of air breathed in and out during regular breathing. CC 0 Public Domain, 2017.
Test your understanding: What is the effect of a snorkel on your breathing? A snorkel is a curved tube used for breathing while you swim with your face in the water. Assume the snorkel is 2 cm in diameter and 40 cm long[11]. See footnote for answer.
One thing you’ll notice about bidirectional breathing is that you breathe in some of the air you just inhaled; roughly 150 mL of air occupies airways that don’t participate in gas exchange.
The respiratory pump in humans and other mammals. Humans and other mammals pump air in and out of their lungs by changing the size of chest (thorax). When the thoracic cavity expands, the lungs expand too. The pressure of the atmosphere pushes air into the lungs, filling them with air. When the thoracic cavity contracts, the air pressure inside the lungs exceeds the atmospheric pressure, and air rushes out of the lungs. That’s how we breathe in, and breathe out.
The lungs are surrounded by tissue membranes called the pleura. The visceral pleura cling to the outer surface of each lung, and together with the parietal pleura lining the thoracic cavity, the pleura form a liquid-filled bag folded over each lung. This arrangement, where two sheets of tissue separated by a layer of liquid surround each lung, lubricates the lungs so that the lungs glide smoothly relative to other parts of the thorax during breathing.
There are two ways that mammals like ourselves can change the size of our thorax to breathe in and out:
- Changing the position of the diaphragm, a muscular, sheet-like barrier that separates the thoracic cavity from the abdominal cavity. Becase the diaphragm is shaped like a dome, contraction expands the thoracic cavity. See Figure 10.19.
- Rotating the ribs up and down. See Figure 10.20.
 Figure 10.19 The diaphragm. Source: O’Followell, Le Corset / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:La_voûte_formée_par_le_muscle_diaphragme.gif Public Domain, 1908.
Figure 10.19 The diaphragm. Source: O’Followell, Le Corset / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:La_voûte_formée_par_le_muscle_diaphragme.gif Public Domain, 1908.
In normal breathing at rest, contraction of the diaphragm does most of the work, roughly three quarters of the work of breathing. When the diaphragm muscles contract, they pull the diaphragm down, increasing the amount of space in the thorax. See Figure 10.18. Air rushes into the lungs. That’s inhalation. When the diaphragm muscles relax, elastic recoil of the lungs return the lungs and diaphragm to where they were before inhalation, pushing the air back out of the lungs. Thus, lungs and other structures of the torso act like springs to store mechanical energy during inhalation, so that moderate exhalation doesn’t take much muscular effort.
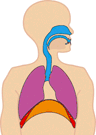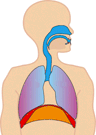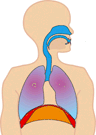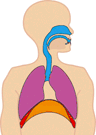 Figure 10.20 The diaphragm is the main pump for breathing at rest. Source: Brbbl / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Diafragma_ademhaling.gif CC BY-SA 3.0, 2007.
Figure 10.20 The diaphragm is the main pump for breathing at rest. Source: Brbbl / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Diafragma_ademhaling.gif CC BY-SA 3.0, 2007.
Expansion and contraction of the ribcage also contributes to normal breathing at rest, but becomes more prominent when there’s a greater need for ventilation. During heavier or deeper breathing, the movement of the ribs becomes more important. Sets of muscles connecting adjacent ribs power these movements. These muscles are called the intercostal muscles. The external intercostals pull neighbouring ribs together, rotating the ribs upwards. Because human ribs slant downwards, raising then expands the thoracic cavity. See Figure 10.22.
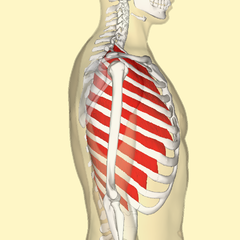 Figure 10.21 Intercostal muscles. Source: The Database Centre for Life Science, Anatomography BodyParts3D, University of Tokyo / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:External_intercostal_muscles_lateral.png and lifesciencedb.jp/bp3d CC BY-SA 2.1 Japan, 2012.
Figure 10.21 Intercostal muscles. Source: The Database Centre for Life Science, Anatomography BodyParts3D, University of Tokyo / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:External_intercostal_muscles_lateral.png and lifesciencedb.jp/bp3d CC BY-SA 2.1 Japan, 2012.
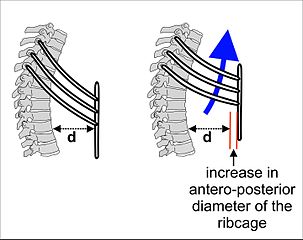 Figure 10.22 The external intercostal muscles help inhalation by rotating the ribs upwards. Source: Cruithne9 / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Ribcage_during_inhalation.jpg CC BY-SA 4.0, 2016.
Figure 10.22 The external intercostal muscles help inhalation by rotating the ribs upwards. Source: Cruithne9 / Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Ribcage_during_inhalation.jpg CC BY-SA 4.0, 2016.
Like the lungs, the ribcage and attached structures show elastic recoil, allowing exhalation to happen passively when the external intercostal muscles relax during moderate breathing.
During even heavier breathing, a deeper set of intercostal muscles become involved. Unlike the external intercostals which expand the ribcage when they contract, the internal intercostals pull the ribs down, allowing more forceful and deeper exhalation of air.
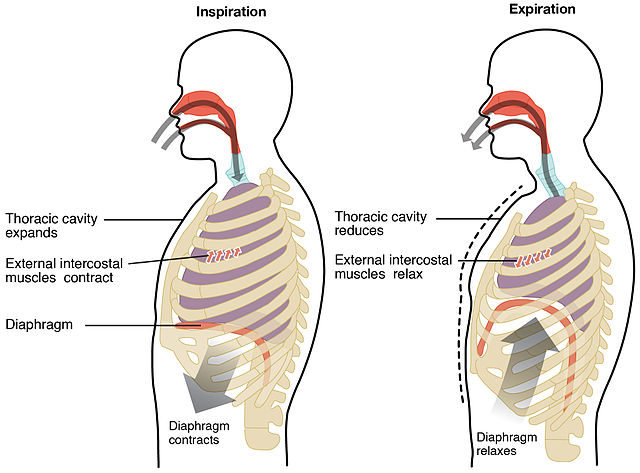 Figure 10.23 Ribcage and diaphragm working together during breathing. Breathing in (inhaling, or inspiration), and breathing out (exhaling, or expiration). Source: Anatomy & Physiology, Rice University, Download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@6.27. CC BY 3.0, 2014.
Figure 10.23 Ribcage and diaphragm working together during breathing. Breathing in (inhaling, or inspiration), and breathing out (exhaling, or expiration). Source: Anatomy & Physiology, Rice University, Download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@6.27. CC BY 3.0, 2014.
Figure 10.24 The diaphragm and ribs in action in a human chest. Source: Biomedizinische NMR Forschungs GmbH am Max-Planck-Institut für biophysikalische Chemie, http://www.biomednmr.mpg.de CC BY-SA 3.0
Why not breathe fast and hard all the time? Can you think of two important reasons why? This is a question that gets to the heart of why there needs to be constant communication between the brain, the respiratory system, and the rest of the body.[12] (See footnote for answer).
Control of breathing
Can you hold your breath? Can you hold your breath until you lose consciousness? Breathing is under both voluntary and involuntary control. The involuntary control systems run constantly from birth until you take your last breath. Voluntary control can override involuntary control for a short time, but if the air in the lungs isn’t refreshed within a few minutes, the involuntary breathing reflexes become overwhelming. It is very difficult for a person to hold his or her breath to the point of passing out.
Most of the time, you breathe without even being aware of your breathing. So why does voluntary control exist? The reasons aren’t immediately obvious. Can you think of a few?[13]
The body’s energy needs vary dramatically from moment to moment. In a typical person, energy consumption varies roughly ten fold between resting and maximum aerobic output. In a trained athlete, energy consumption can increase twenty fold during a burst of strenuous activity. The rate of gas exchange needs to be closely matched to these changes – involuntary control of breathing matches the ventilation rate to the metabolic demands of the body.
Can you think of three different chemical signals cells can use to monitor the rate of cellular respiration in the rest of the body?
If you guessed the following three, you’re right:
- Partial pressure of oxygen in body fluids.
- Partial pressure of carbon dioxide in body fluids.
- pH (concentration of hydrogen ions) in body fluids.
It might come as a surprise, but pH is the most important of the three in normal control of breath rate, but only as a measure of the carbon dioxide concentration in the blood. An increase in energy use by the body produces excess carbon dioxide which – unlike bicarbonate and hydrogen ions – can pass easily from the blood to the cerebrospinal fluid. Carbonic anhydrase in and around the medulla converts this carbon dioxide to bicarbonate and hydrogen ions. Neurons in the brainstem, specifically in the medulla (see Figure 10.25) detect the drop in pH, and increase the breath rate and depth of breathing accordingly. These clusters of neurons are called the central chemoreceptors. The central chemoreceptors respond to oxygen as well, but oxygen only becomes important in the regulation of breathing when blood oxygen drops down below a substantially hypoxic level of 60 mmHg.
There are also chemoreceptors that sense the blood directly for oxygen, carbon dioxide, and pH. These are called the peripheral chemoreceptors. They are located in the wall of the inner carotid arteries supplying the brain, where they form part of the carotid bodies, and in the wall of the largest artery in the body, the aorta, where they form part of the aortic body.
The carotid bodies send signals to the medulla through the glossopharyngeal nerve (cranial nerve IX), while the aortic body sends signals to the medulla through the vagus nerve (cranial nerve X).
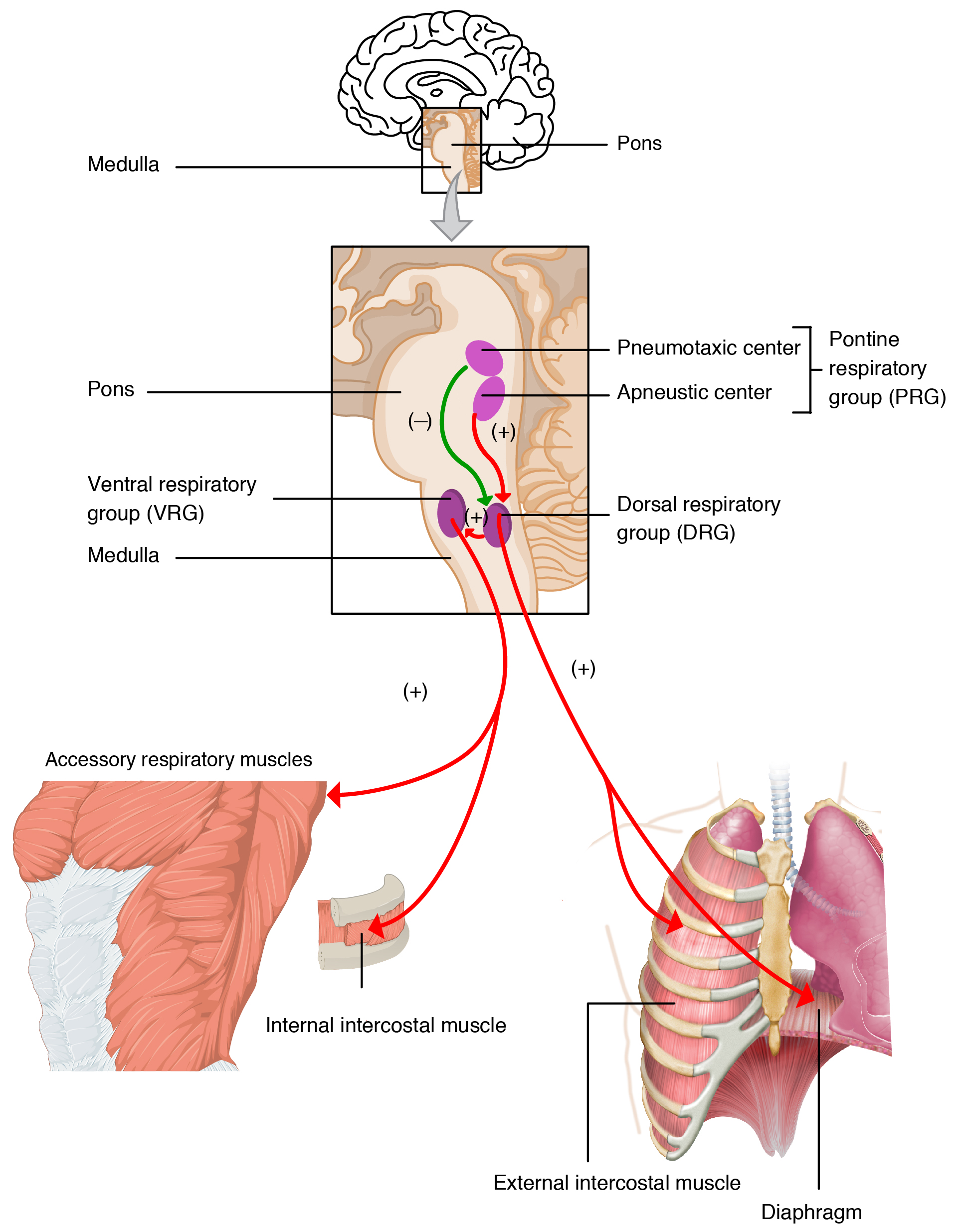 Figure 10.25 Brainstem control of breathing. Source: The Process of Breathing, Rice University, Download for free at http://cnx.org/contents/bbaedbf4-4d78-4b7c-bc94-2a742f0f2f8c@6. CC BY 3.0, 2013.
Figure 10.25 Brainstem control of breathing. Source: The Process of Breathing, Rice University, Download for free at http://cnx.org/contents/bbaedbf4-4d78-4b7c-bc94-2a742f0f2f8c@6. CC BY 3.0, 2013.
Test your understanding: Many freedivers breathe hard and fast (hyperventilate) at the surface, just before going underwater. Does hyperventilating beforehand make diving more safe, or less safe?[14] See footnote for the answer.
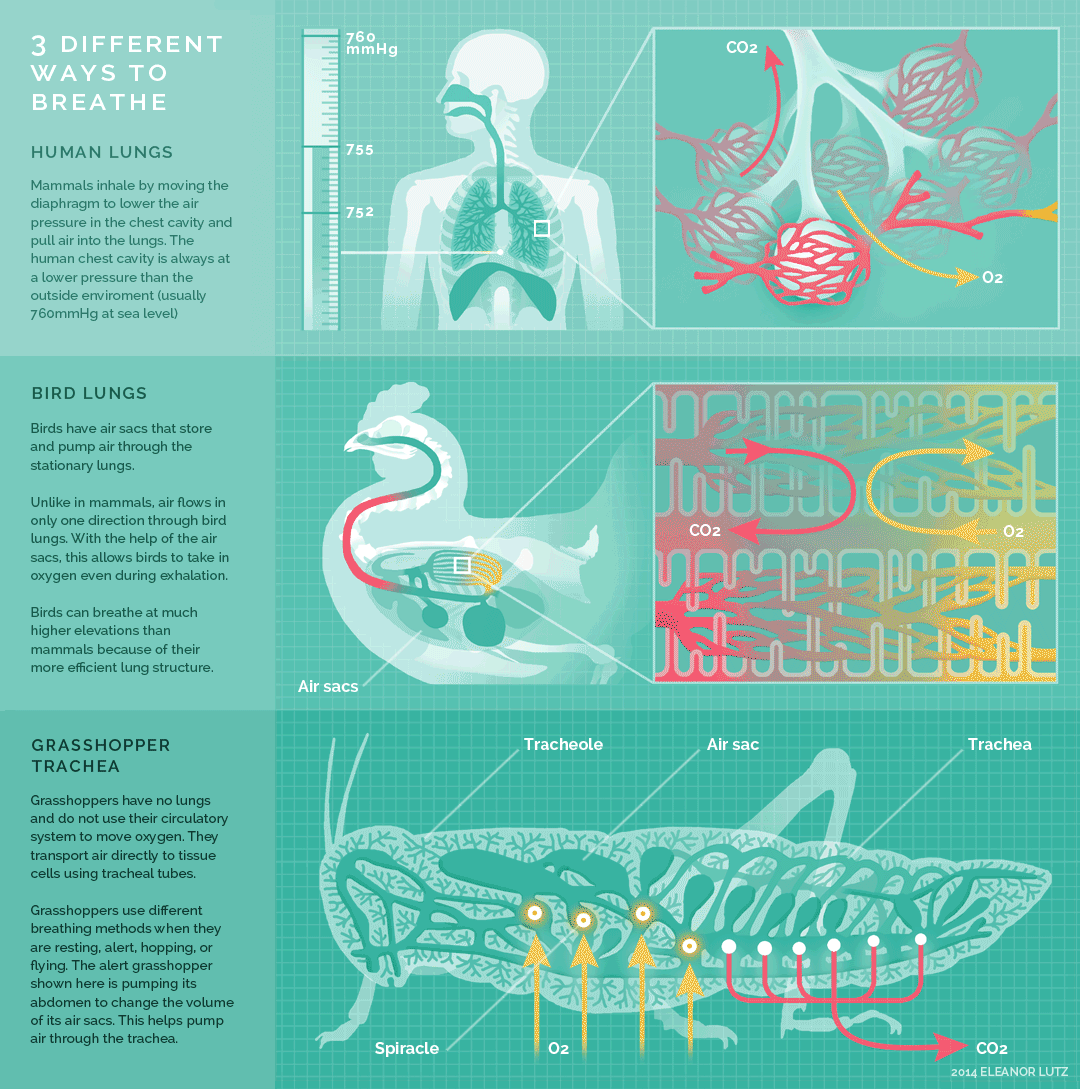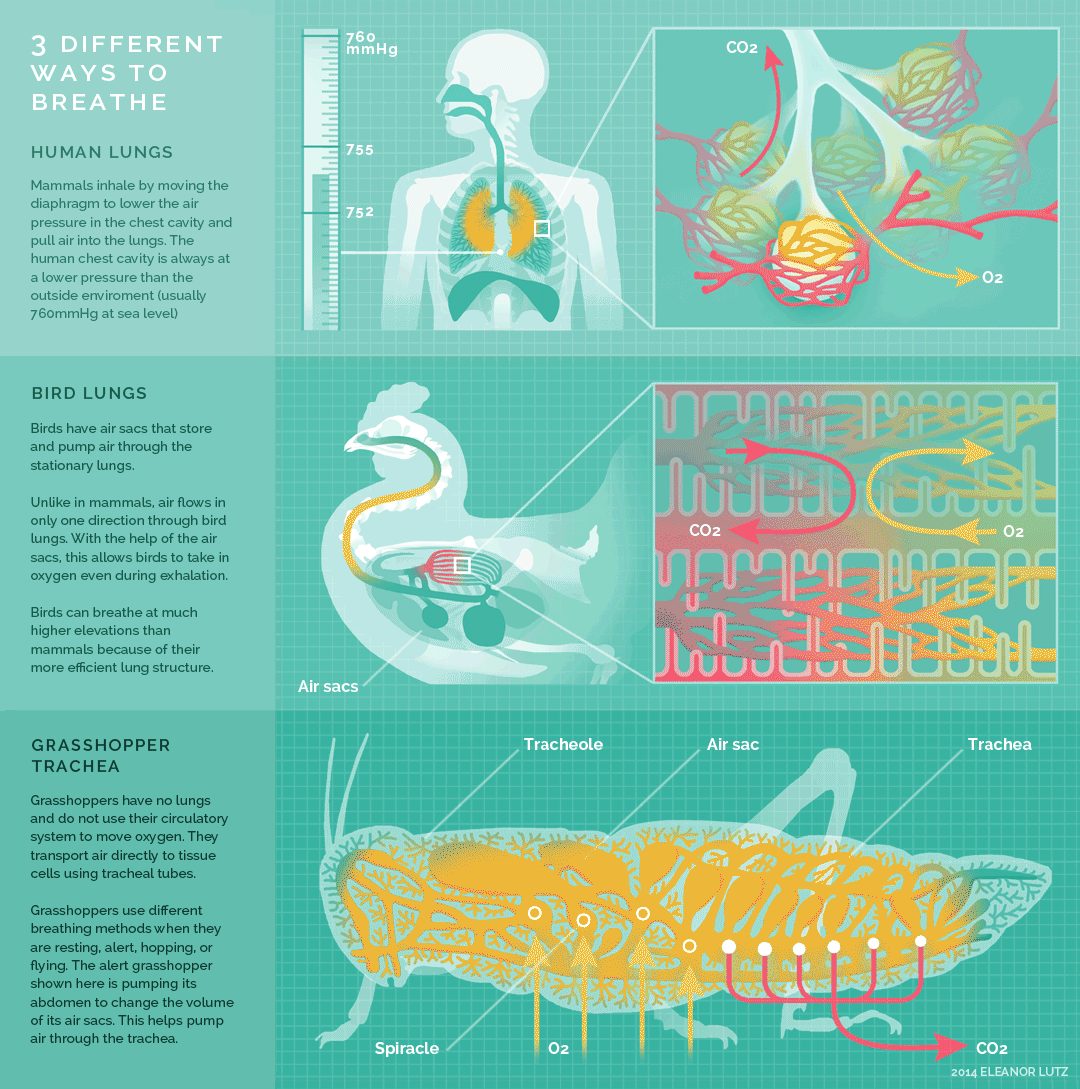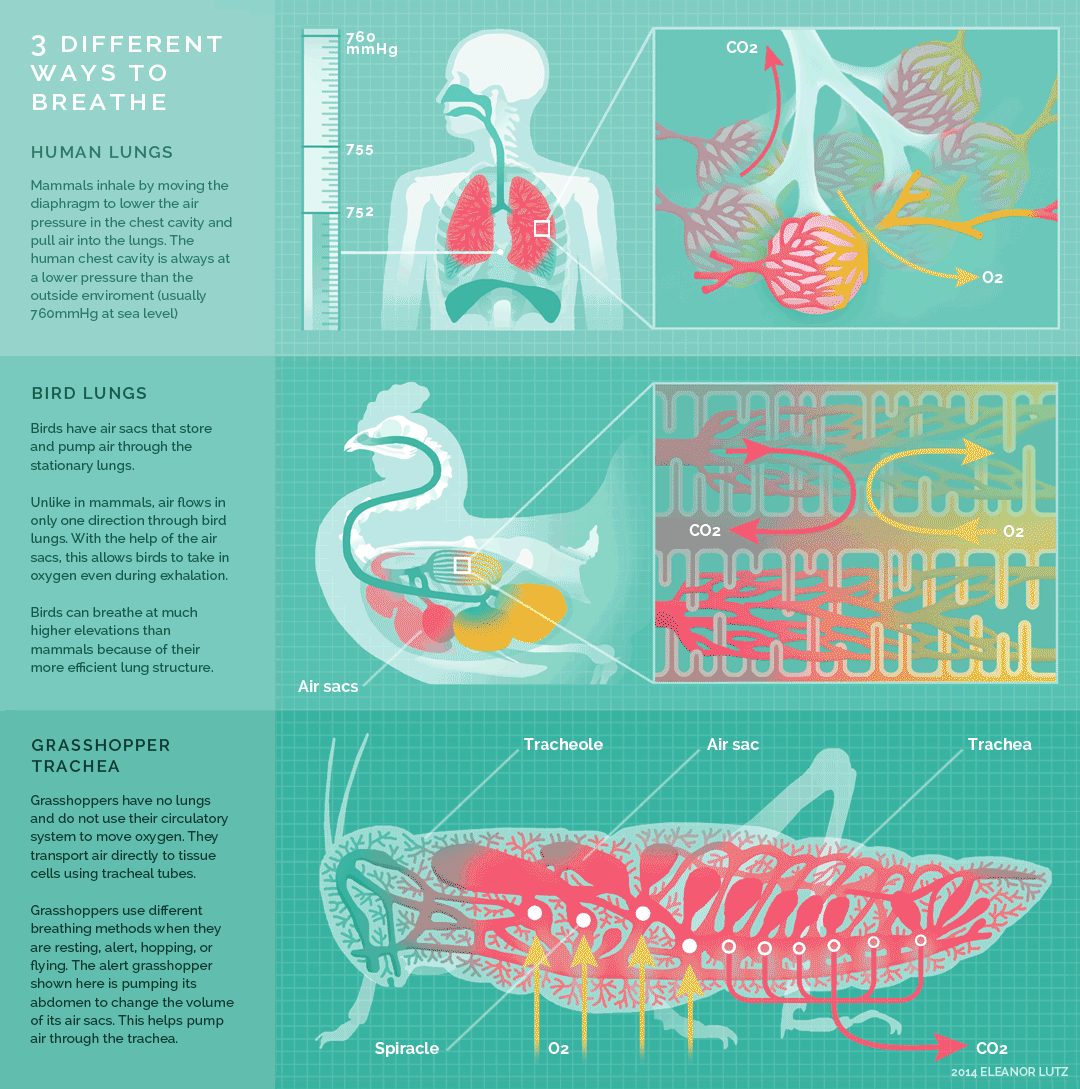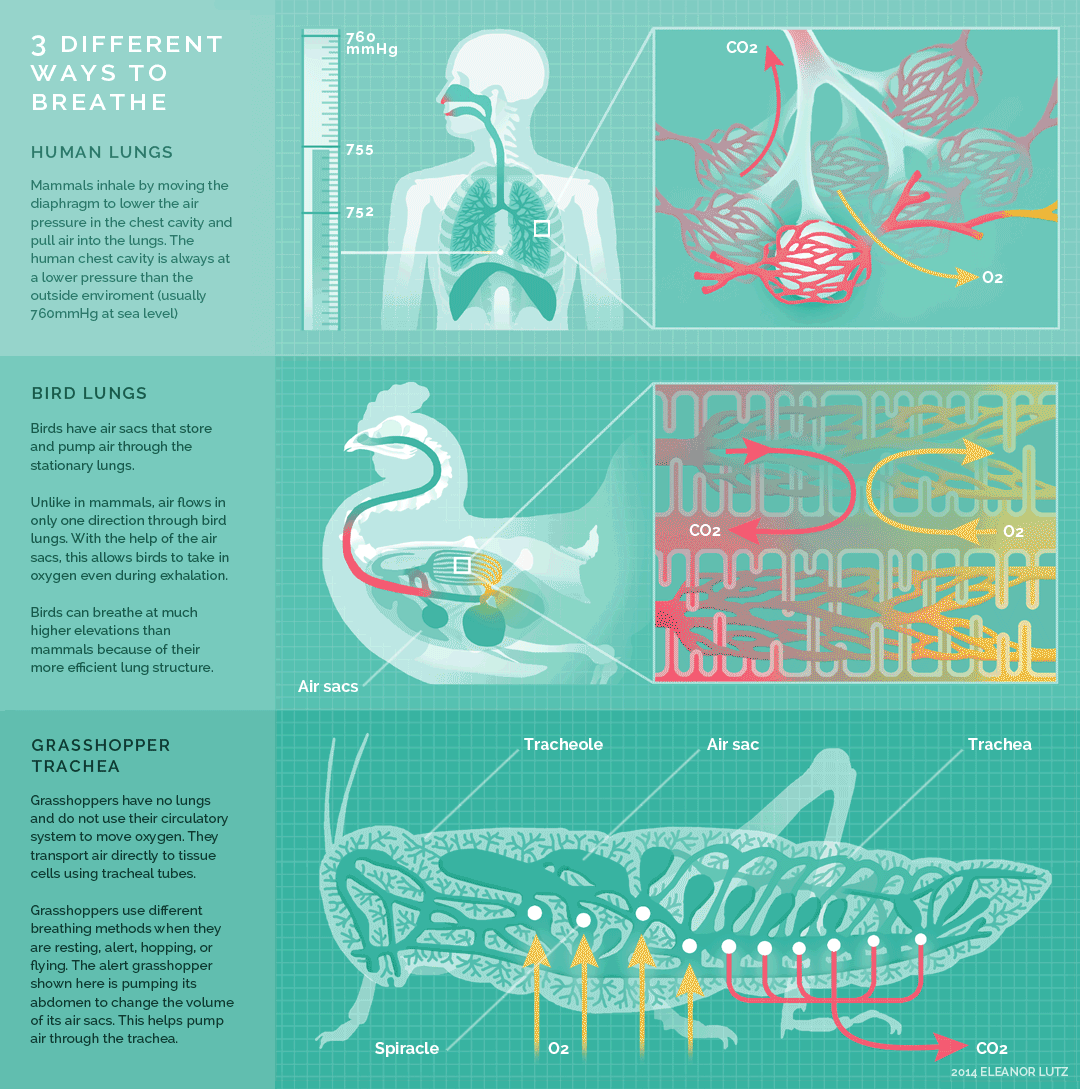 Figure 10.26 Summary of breathing in humans, birds, and insects. Source: Eleanor Lutz, http://tabletopwhale.com/2014/10/24/3-different-ways-to-breathe.html CC BY-NC-ND 4.0, 2014.
Figure 10.26 Summary of breathing in humans, birds, and insects. Source: Eleanor Lutz, http://tabletopwhale.com/2014/10/24/3-different-ways-to-breathe.html CC BY-NC-ND 4.0, 2014.
10.7 Suggested Readings
The Respiratory System, in Anatomy & Physiology, OpenStax CNX.
https://cnx.org/contents/FPtK1zmh@8.83:GJrqqsRC@7/Introduction
from Anatomy & Physiology, OpenStax CNX. June 13, 2017 http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.83
10.8 Glossary
Acidosis – The blood is more acidic than normal, meaning the concentration of hydrogen ions is high, or equivalently, the pH is low. Blood pH below about 7.35 is typically considered to be acidosis.
Air – The gas mixture that we normally breathe. Air is 78% nitrogen (N2), 21% oxygen (O2), and 0.04% (until recently, less than 0.03%) carbon dioxide (CO2).
Alkalosis – The blood is more alkaline than normal, meaning the concentration of hydrogen ions is low, or equivalently, the pH is high. Blood pH over 7.45 is typically considered to be alkalosis.
Alveolus (plural, alveoli) – Thin-walled, tiny air sac in the lung. Alveoli are where gas exchange takes place, and the human lung contains hundreds of millions of alveoli.
Anoxia – The absence of molecular oxygen (O2).
Aortic body – Small cluster of sensory neurons and supporting cells embedded in the wall of the aorta, the largest artery. The neurons are chemosensory, responding to pH, oxygen levels, and carbon dioxide levels.
Apneustic centre – A set of neurons in the pons which signal to the medulla to promote inhalation.
Asthma – A long-term inflammatory inflammatory disease of the airways. Asthma sufferers sometimes experience bronchiole smooth muscle spasms, causing the bronchioles to narrow, impeding air flow. This is called an asthma attack.
Atmosphere – The thin film of gas clinging to the surface of the Earth. See air.
Boyle’s law – For a given number of gas molecules (amount of gas) and temperature, pressure times volume stays constant. In other words, gas pressure is inversely related to volume. P1V1 = P2V2.
Breathe – Pump air bidirectionally, letting air into the body, then pushing it back out again.
Bronchiole – Bronchioles are smaller airways branching from the bronchi.
Bronchitis – Inflammation of the bronchi.
Bronchus – Rigid tube that acts as an airway, connecting the trachea with one lung. The plural of “bronchus” is “bronchi”.
Capillary – The narrowest blood vessels, only 5 to 10 microns across. Their walls are extremely thin, allowing exchange of many substances between the blood and the surrounding tissues.
Carbon dioxide – CO2.
Carbonic anhydrase – Enzyme that greatly speeds up the interconversion of carbon dioxide with bicarbonate in water:
CO2 + H2O ↔ HCO2– + H+
Carotid body – Small cluster of sensory neurons and supporting cells embedded in the wall of the internal carotid arteries, which supply blood to the brain. The neurons are chemosensory, responding to oxygen levels, carbon dioxide levels, and pH.
Cilium – A short extension of a cell, containing a bundle of microtubules. Co-ordinated beating motion of cilia propels extracellular fluid across the surface of a cell.
COPD – Chronic Obstructive Pulmonary Disease. See bronchitis and emphysema.
Cough – A reflex that propels a sudden burst of air out of the upper respiratory tract, out of the mouth.
Countercurrent – Neighbouring currents flow in opposite directions. Results in highly efficient transfer of materials between two fluids by diffusion.
Cyanosis – A slightly blue colour of mucous membranes and skin due to high deoxyhemoglobin levels. Cyanosis becomes noticeable when blood oxygen is less than roughly 70-80% of saturation.
Dalton’s law – The partial pressure exerted by each of the gases in a gas mixture add up to the total pressure exerted by the gas mixture.
Dead space – The portion of air in the respiratory tract that does not significantly exchange gases with the blood with each breath. The dead space is equal to the volume of the conducting airways.
Deoxyhemoglobin – Hemoglobin without O2. See cyanosis and oxyhemoglobin.
Diaphragm – Muscular barrier between the thoracic cavity and the abdominal cavity. The diaphragm’s main function is to power breathing, but it helps with other functions, such as defecation and vomiting.
Dilation – Increase in inner diameter, for example when air passages or blood vessels expand to get wider. Dilation of a tube lowers resistance to flow.
Cystic fibrosis – A disease due to a defect in a chloride channel. Results in unusually thick mucus that obstructs breathing.
DRG – Dorsal Respiratory Group, a group of neurons in the medulla which respond to inputs from the peripheral chemoreceptors and lung stretch receptors to trigger inhalation.
Elastic – Able to store mechanical energy when stretched, such that the material passively returns to its original shape when the stretching force is no longer present.
Elastic recoil – Return to original shape after external force is removed. See elastic.
Emphysema – Lung disease where the alveolar walls break down, leading to large air spaces. See COPD.
Energy – In physics, defined as a quantity that cannot be created or destroyed; energy and mass are the same thing. In biology, energy is more narrowly defined as the practical capacity to do mechanical work and generate heat.
Epiglottis – A flap of tissue that closes off the airway during swallowing, so that food and drink don’t enter the larynx..
Epithelium – A sheet of cells lining the inner or outer surface of various organs, glands, and vessels. One of the basic tissue types of the animal body.
Expiration – Exhalation; breathing out.
Globin – Protein of a diverse family of O2-binding proteins that cotain heme and iron.
Gill – Structure for gas exchange with water from the environment.
HACE – High altitude cerebral edema.
HAPE – High altitude pulmonary edema.
Heme – The oxygen-binding cofactor in globins such as hemoglobin. Contains an atom of iron.
Hemocyanin – A blue protein that carries O2 in the hemolymph of molluscs, crustaceans, and some spiders, scorpions, and centipedes. Hemocyanins are unrelated to globins, and contain copper, not heme and iron.
Hemoglobin – Abundant globin of red blood cells, the main carrier of O2 in many animals.
Henry’s law – At equilibrium, the concentration of a dissolved gas in a liquid is proportional to the partial pressure of the same gas in the gas phase.
Hering-Breuer reflex – The reflex that prevents over-inflation of the lungs. Stetch receptors in the lung signal through the vagus nerve to the medulla and pons.
Hypercapnea – An abnormally high concentration of carbon dioxde in the blood.
Hypoxia – Lower than normal oxygen level in the body.
Inspiration – Inhalation; breathing in.
Intercostal muscle – Muscle connecting neighbouring ribs. The main function of the intercostals is to power breathing.
Lactate or lactic acid – A low molecular mass organic acid that’s the product of anaerobic muscle action.
Larynx – Structure at the entrance to the trachea. The larynx helps the epiglottis keep solids and liquids out of the trachea during swallowing, closes and opens to produce the rush of air in the cough reflex, and produces sound for communication between individuals.
Lung – Air-filled, elastic bag with a large surface area for gas exchange with the blood.
Lung compliance – The change in lung volume for a given change in pressure. Low lung compliance makes breathing difficult.
Medulla – Portion of the brainstem that controls several vital functions of the body, including breathing.
Mucus – Slimy, slippery, gel-like liquids produced by mucous membranes of the respiratory, digestive, and reproductive tracts. Characterised by high molecular weight glycoproteins dissolved in water.
Myoglobin – A globin found in muscle cells.
Nitric oxide – NO.
Oxygen – O2.
Oxyhemoglobin – Hemoglobin loaded with O2.
Partial pressure – The pressure exerted by one gas in a gas mixture. See Dalton’s law. Also a measure of the concentration of a gas dissolved in a liquid. See Henry’s law.
Phlegm – Mucus expelled by coughing.
Pleura – Two thin sheets of tissue surrounding each lung, the inner (visceral) pleura covering the lung, and the outer (parietal) pleura attached to the chest wall. The liquid contained between the pleura lubricate and cushion the lungs. The pleura are epithelia derived from the mesoderm.
Pneumotaxic centre – Clusters of neurons in the pons which signal to the medulla to inhibit inhalation.
Pneumothorax – One of those situations when duct tape comes in handy. Also known as a sucking chest wound. See Transpulmonary pressure.
Pons – The part of the brainstem between the medulla and the midbrain. The pons includes clusters of neurons in the pneumotactic centre and apneustic centre, which communicate with the medulla to adjust the rate and depth of breathing.
Pulmonary artery – Artery that supplies blood to the lungs. Unlike other arteries, the pulmonary arteries carry deoxygenated blood.
Respiration – Breathing.
Respiration – Exchange of oxygen and carbon dioxide between a living thing and its environment.
Respiration – Release of stored energy in cells from oxygen and organic fuel. Mitochondria are the site of respiration in animal cells.
Spiracle – Opening through which insects breathe.
Surface tension – Tension at the interface of water with another fluid, such as air or oil. Due to cohesion through hydrogen bonds, pure water has high surface tension.
Surfactant – Any substance that reduces surface tension.
Thorax – The portion of the trunk from the diaphragm to the neck, surrounded by the rib cage.
Tidal volume – The volume of air inhaled and exhaled in one breath.
Trachea – Rigid pipe that acts as an airway, connecting the larynx to the bronchi.
Tracheae – Air vessels that insects use to breathe.
Transmural pressure – The difference in pressure between two sides of a barrier.
Transpulmonary pressure – The difference in pressure between the air in the alveoli – usually close to atmospheric pressure – and the fluid surrounding the lungs – usually a little bit below atmospheric pressure. Transpulmonary pressure is what keeps the lungs inflated with air. See Transmural pressure.
Ventilation – Bringing a flow of air. See breathing.
Vocal cord – Flap or fold of tissue in the larynx that helps produce sound.
- People often survive up to two months without food, if they can stay warm. ↵
- In hot weather, less than an hour. At a comfortable temperature, around two weeks. ↵
- World records for holding one’s breath tend to be around ten minutes. ↵
- If a planet’s atmosphere contains molecular oxygen (O2), that would be a likely sign of life. Oxygen is greedy for electrons. In other words, it's a strong oxidizer. As a result, it reacts with a wide range of substances. Oxygen would vanish from the atmosphere if it isn't constantly replenished by photosynthesis. ↵
- A few animals that live in the desert can survive without drinking, by conserving metabolic water generated by cellular respiration. Those migrating birds that fly thousands of kilometres without stopping also rely heavily on metabolic water. Burning of fats produces a roughly equivalent amount of water. ↵
- The brain is 2% pecent of the human body’s mass but consumes 20% percent of the body’s energy. Maintaining ion concentrations – low Na⁺ and high K⁺ inside the axon – to keep the membrane potential “charged” takes a lot of energy. The Na⁺/K⁺-ATPase alone consumes roughly a third of a neuron’s energy budget. Maintaining synapses in a state ready to release neurotransmitter also takes a lot of energy. ↵
- One kilocalorie is one thousand calories. Confusingly, “calories” listed on food labels in Canada and the US are in fact kilocalories, so one “food calorie” is one thousand calories, or 1 kcal. ↵
- One Watt is one Joule per second, equivalent to approximately 0.24 calories per second. ↵
- Unlike ions, oxygen dissolves relatively well in the hydrophobic lipid part of biological membranes, so cell membranes are no barrier to diffusion of oxygen molecules. ↵
- Whales, porpoises, and dolphins are an exception, because they spend their entire lives in the water. Their nostrils are one or two blowholes near the top of the head. Relocating the nostrils or single fused nostril up and back makes breathing easier when surfacing to get air. ↵
- You’d have to breathe deeper than normal to achieve the same rate of gas exchange, because the air in the snorkel contributes to the respiratory dead space. The swimmer breathes in air that remained in the snorkel from the prior breath out. The volume of the snorkel is pi × (1 cm radius)2 × 40 cm length = 125 mL, nearly doubling the respiratory dead space in a typical adult. There would also be an increase in airway resistance. ↵
- One reason is that the mechanical work of breathing expends a substantial amount of energy, so it doesn’t make any sense to breathe more than necessary. Even at rest, the work of breathing accounts for 4% or 5% of the total oxygen consumption in humans, mostly to power the diaphragm. This is a significant evergy cost. During strenuous exercise, when your intercostak muscles are fully engaged, the muscular work of breathing can exceed your body's total energy use at rest. The other reason why breath rate and depth varies is that hyperventilating (breathing harder than needed for gas exchange) blows off more carbon dioxide than normal, which results in a high blood pH (alkalosis), disrupting the normal homeostasis of blood pH. ↵
- In many vertebrate animals, the respiratory system has a second function: communication by sound. This is especially important in humans, who use fine control of breath for speaking and singing. Another situation where voluntary control is important is holding one's breath for diving underwater. ↵
- Hyperventilating before diving makes diving much more dangerous. The haemoglobin in your arterial blood is already close to 100% saturated with oxygen, so hyperventilating won’t increase the amount of oxygen in your body. But hyperventilating will substantially reduce the amount of carbon dioxide in your body. This delays the urge to breathe, because breathing is mostly controlled by blood carbon dioxide levels. Blood oxygen levels can drop dangerously low, risking unconsciousness before enough carbon dioxide can build up to encourage the diver to come up to breathe. This is called “shallow water blackput”. Many people have drowned in very shallow water for this reason, because they mistakenly hyperventilated before diving. ↵

