Digging In
6 Soil Biodiversity and Ecology
Nathan Basiliko; Kari Dunfield; and Mario Tenuta
LEARNING OUTCOMES
On completion of this chapter, students will be able to:
- List and describe the major types and groups of biota that live in soils, including their size, distribution, and taxonomy
- Define the metabolic strategies of soil biota in terms of how they harness energy and nutrients from the environment
- Describe some of the major challenges and technological breakthroughs in how we study and learn about soil biota
- Describe how biological communities are integral drivers of organic matter turnover, nutrient cycling, and soil fertility that are covered in detail in Chapters 3 and 7
- Describe some of the important trophic interactions (i.e., who eats whom) among biotic groups in soils
INTRODUCTION
The mysterious community beneath your feet!
Since Schatz and Waksman’s 1943 discovery of the lifesaving antibiotic streptomycin produced by soil bacteria, soil biology has grown to be one of the most exciting and cutting-edge disciplines. Microbiologists have gone on to discover numerous other vital classes of antibiotics produced by soil microbes. Have you ever had a bacterial or fungal infection and been prescribed an antibiotic?- thank soil microbes. But those discoveries only begin to scratch the surface of the value of the biotic communities that make soils their home. Soils are now recognized as arguably the most biodiverse habitat on Earth. The exceedingly complex physical and chemical makeup of soils you encountered in Chapters 3-5 has given rise to vast niche diversity and, thus, the evolution of millions, if not billions, of unique taxa of soil biota. These communities dynamically shape the soil environment, and in the process influence such vitally important ecosystem services ranging from plant and crop growth and health, to water quality, even the regulation of the global climate system through the storage of atmospheric carbon and exchange of atmospheric greenhouse gasses.
Can You Dig It!

As unlikely as it may sound, a soil scientist won the Nobel Prize in Physiology or Medicine for curing tuberculosis, once one of the world’s most devastating respiratory diseases. Ukrainian born Selman Waksman spent most of his career as a professor at Rutgers University in New Jersey (U.S.A.), and was a pioneer in the early 20th century studying soil microbiology and organic matter.
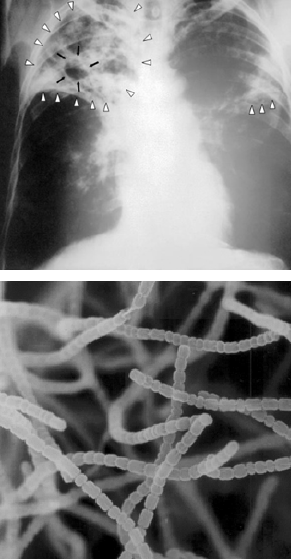
His group was one of the first to tackle the complex chemical nature of soil humus (see Chapter 3), including how soil microorganisms play roles in its formation and decay. Waksman’s research group also focused on the unique physiological properties of the fungi and bacteria they were culturing from soils, including that some produced powerful antibiotics.
Ph.D. student Albert Schatz was supervised by Waksman and characterized Waksman’s large collection of Streptomyces cultures obtained from local soils and composts for the ability to kill the pathogen Mycobacterium tuberculosis, which led to eventual isolation of streptomycin, the first antibiotic that effectively killed the pathogen without also killing the patient! The story goes that, according to Schatz, Waksman was too afraid to work with the Mycobacterium cultures for which there was no cure at the time, and never ventured to the lab where Schatz was handling the TB-causing microbe. The pair published on the seminal discovery of streptomycin together- and streptomycin was later produced commercially saving countless lives. This discovery was not without controversy, however, as Schatz was initially shut out of any patent royalty payments, and the 1952 Nobel Prize in Physiology or Medicine was awarded solely to Waksman. The apparent rationale was that the difficult task had been obtaining the extensive soil bacteria cultures, while the selection procedures to test for antibiotics were more routine.
In this chapter we introduce the range of soil organisms, from sub-micrometer diameter (that is, less than 1/1,000,000 m) unicellular bacteria and archaea to the much-loved (but also occasionally loathed) night-crawler, to vertebrate fauna. What are their strategies for survival and growth, and how do they interact with each other? How do soil microbes and fauna dynamically interact with their physical environment (building on Chapter 4) and with plants? We will explore soil biota as key players driving the cycling of carbon and organic matter you read about in Chapter 3, and the cycling of essential nutrients like nitrogen that you will read more about next in Chapter 7.
An important theme will emerge in this chapter: we presently know very little about the breadth and depth of soil biodiversity, nor do we understand the exact functions of many populations and communities. This is especially true for the soil microorganisms and microarthropods, however, in the past decade, molecular biology tools for detecting and characterizing biodiversity have begun to give us a picture of the millions to billions of “known unknown” organisms dwelling in soils. This makes soil biology an exciting, breakthrough field; however, we can almost certainly guarantee that if you are reading this chapter 10 years or so from now, at least some of what we now “know” will have changed!
SOIL BIODIVERSITY
Who lives in the soil?
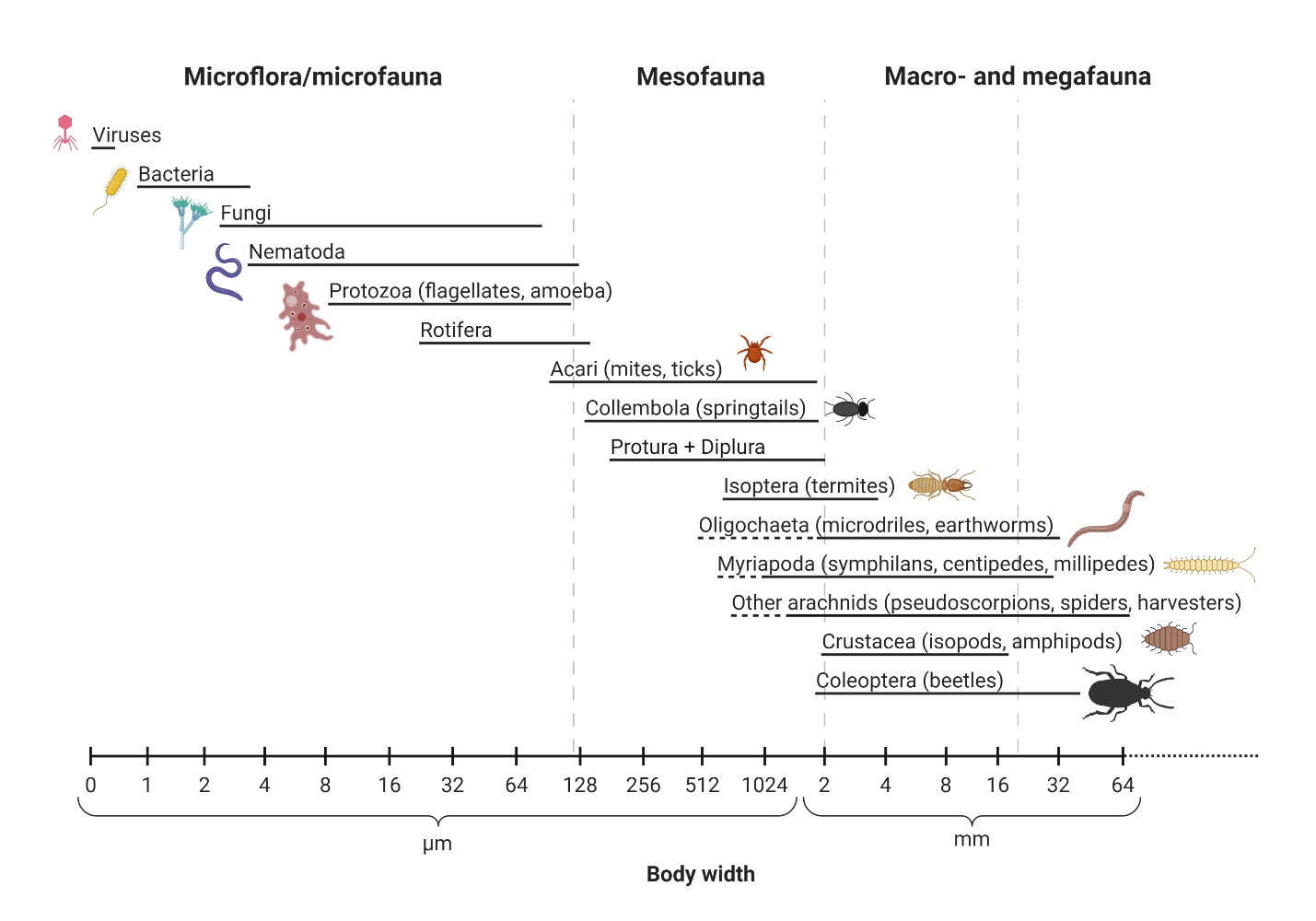
One small handful of healthy surface soil contains more organisms than the whole population of humans on Earth, up to 10 billion individual cells in a single gram! Soil biota range from microscopic unicellular bacteria and archaea that lack nuclei and other organelles to warm-blooded borrowing small mammals like voles and woodchucks, as well as the hidden half- give or take- of plants. The range of sizes of organisms in soil ecosystems spans across nearly ten orders of magnitude! For example, the smallest soil bacterium is ~0.5 micrometers in diameter (0.000005 m), soil-dwelling amphibians and mammals might typically be ~10 cm long (0.1 m), plant roots are typically in the 0.1 to 10 m length range, while certain pathogenic soil forest soil fungi grow thread-like mycelia that extend up to 1 km (1000 m) and beyond! Traditionally soil scientists have lumped soil biota together roughly by some combinations of size and function (see Figure 6.1). Although it is useful to be aware of these groups because the terminology still is in use, here we introduce broad groups of soil biota a bit differently, beginning with the prokaryotes, that are the bacteria and archaea. The term prokaryote refers to single cellular organisms that lack internal membrane-bound organelles (e.g., nucleus, mitochondria, chloroplast), while eukaryote refers to organisms whose cell(s) contain(s) at least a nucleus, and often times other organelles.
Soil scientists and ecologists often use the following groups to describe soil biota:
- Microflora: bacteria and archaea (the two domains of life termed prokaryotes, that is, organisms lacking nuclei and other organelles), algae and fungi.
- Macroflora: plant roots.
- Microfauna: protozoa (single cellular eukaryotes that are motile), small nematodes, and small arthropods.
- Mesofauna: metazoans (referring to the animal phylum) with body sizes typically between 0.1 and 2 mm as well as the larger predatory and omnivorous nematodes.
- Macrofauna: all metazoan invertebrates and members of the chordate phylum (mammals, amphibians) larger than 2 mm.
However, this scheme can be confusing. For example, whereas the group name microflora implies “little plant”, only the algae and cyanobacteria are – like plants – photoautotrophs while many soil bacteria and all soil fungi are heterotrophs. In another example, although certain fungi (yeasts) are unicellular, others form large macroscopic (to massive) fruiting bodies and mycelia (see Figure 6.2).

Bacteria and archaea
Being similar in size (typically just a one to a few micrometers in diameter), single cellular, and lacking organelles, it took until the early 1990s before bacteria and archaea were widely recognized as being clearly distinct groups from one another. Although they share some traits, bacteria and archaea diverged from each other evolutionarily a very long time ago. This divergence led to bacteria and archaea having fundamentally different cell envelopes (cell membranes, cell walls, and other outer layers), protein synthesis machinery, mechanisms for packaging their DNA, among many other major physiological differences. In fact, based on DNA sequence analyses, they are as dissimilar to each other as each of them is to higher plants or animals! Carl Woese from the University of Illinois (U.S.A) was the principal scientific force behind the first major redrawing of the tree of life (showing relatedness among all organisms) based on DNA sequences and proposed just three main branches representing the domains Bacteria, Archaea, and Eukarya. Despite their fundamental differences, both bacteria and archaea are small and can interact with micro-scale habitats in soil particles, and both have the ability to evolve quickly to new environments, conditions, and resources with common mechanisms of horizontal gene transfer. This means that they can have some niche overlap in soil environments, and a diverse range of metabolic strategies exists across both domains (Table 6.1), including:
- source of energy (ATP);
- source of molecules to provide electrons to the electron transport chain to produce metabolic energy as ATP; and
- source of carbon to build new organic molecules.
In surface soils, bacteria predominate over archaea in number and biomass. However, there are exceptions where lithotrophic archaea carry out very specific reactions of ammonia oxidation (see Chapter 7) in the nitrogen cycle, or producing methane in flooded, oxygen-free soils. The roles of many other soil archaea are not well known. The interesting strategy of obtaining energy (electrons) from the environment through lithotrophy literally means “eats mineral matter” (see Table 6.1).
Table 6.1. Common strategies (and related terminology, all ending with “-troph” roughly meaning food) for soil biota to acquire energy for ATP generation and carbon to build biomass. Note that only certain bacteria and archaea are lithotrophs, acquiring their electrons for redox and ATP generation from reduced mineral compounds
| Source of energy/electrons | Source of carbon for building biomolecules | Termed: |
|---|---|---|
| Sunlight harnessed to split H2O | CO2 (inorganic carbon is chemically reduced or “fixed” to organic forms using the Calvin Cycle) | Photoautotroph (phototroph) |
| Reduced mineral (i.e., non-organic) compounds | CO2 (inorganic carbon ‘fixed’ to organic forms using the Calvin Cycle or other assimilation pathways | Chemolithoautotroph |
| Reduced mineral compounds | Organic matter | Chemolithoheterotroph |
| Organic matter | Organic matter | Chemoorganoheterotroph (heterotrophs) |
Many soil bacteria are heterotrophic (see Table 6.1). Most utilize organic carbon molecules that enter the soil ecosystem mainly as plant litters or from plant roots and are already in a chemically-reduced state (that is, carbon is in reduced form as organic molecules as opposed to carbon dioxide). Cyanobacteria that are commonly found in marine and aquatic habitats can also inhabit wet surface soil environments exposed to sunlight. Cyanobacteria are also known to live symbiotically and in mutualistic relationships with a number of plant species. Some lichens on the surface of soils also contain cyanobacteria as the photosynthetic symbiont. Unlike eukaryotic organisms, a good proportion of soil bacteria and some archaea have the ability to carry out cellular respiration in the absence of molecular oxygen (O2), instead utilizing oxidized forms of other elements like nitrogen (N), sulfur (S), manganese (Mn) and iron (Fe) as the terminal electron acceptor in the production of ATP (the main energy currency in cells). These anaerobic respiration pathways often generate relatively more ATP than fermentation reactions, which certain eukaryotes like yeasts can carry out when O2 is lacking, but also dramatically influence nutrient budgets in soils. Because of their small size, bacteria and archaea can reside in soil pores as small as 1 micrometer in diameter. Many are attached to the soil matrix, however, some remain motile, moving around and between particles via films of soil water.
Fungi
Soil fungi (generally divided into categories based on form as single cellular yeasts and multicellular, filamentous molds) are heterotrophs, more specifically, eukaryotic chemoorganotrophs (see Table 6.1). They play a very important role in the decomposition of woody and other cellulose and lignin-rich plant litters that are low in nutrients like nitrogen (see Chapter 3). Thus, they tend to dominate decomposition processes over bacteria in many forest soils, whereas bacteria may become dominant in more near-neutral pH and alkaline soils. Together, fungi and bacteria are often regarded as the main heterotrophic microbial decomposers in soils. Although fungal cell numbers are generally lower than bacteria in soils, their cell sizes are typically about ten times as large, and in many cases, the contribution to decomposition rates can be about equal between bacteria and fungi in slightly acidic soils. Fungi typically can withstand lower water availability (Aw; Chapter 4), while certain bacteria can outperform fungi in low- and no-oxygen (anoxic, also called anaerobic) conditions.
Certain fungi, called mycorrhizal fungi live symbiotically with most plant species (collectively the fungi and the plant hosts are called mycorrhizae). Specific mycorrhizal fungi are able to colonize the roots of specific plant species and extend their filamentous tufts of hyphae (the larger, often quite-macroscopic structures of hyphae are called mycelia), and at first glance might look like plant roots themselves (Figure 6.3). Usually, the association is mutualistic in mycorrhizae, where the plant supplies sugars as sources of energy to the fungal partner, and in turn, the fungus aids the plant in acquiring nutrients from soil, warding off would-be pests or pathogens with antibiotic defense compounds, and helping improve soil structure (see Chapter 4) in the rhizosphere (i.e., the zone near plant roots) through the secretion of sticky protein and polysaccharide compounds. There are different types of mycorrhizal associations, and these are plant-specific, including ectomycorrhizal fungi that surround the outer layers of cells of plant roots, and arbuscular mycorrhizae that are generally obligate symbionts of plants (i.e., they can’t grow without their plant host) and that extend through cell walls of the root cortex (Figure 6.3). Mycorrhizal mycelial networks in soils can be pervasive and connect roots from multiple plants- and occasionally between multiple plant species, and facilitate energy (sugars) and nutrient (nitrogen and phosphorus) flow between them! A soil internet of sorts.

The life cycles of different soil fungi vary. Yeasts remain unicellular but can exist in either (1n) haploid or (2n) diploid forms under different conditions and divide through mitosis in either 1n or 2n state. The 1n body form is the main state of fungi, with the 2n state being very small and present only during sexual reproduction (genetic combination) when conditions are poor (e.g., moisture stress) or resources, like readily decomposable plant materials, are scarce. Introducing genetic variations might allow them to cope better with stress. The 2n state undergoes meiosis to return the fungus to the 1n haploid state. Stages of multicellular filamentous mould lifecycles can include fruiting body phases (mushrooms, puffballs) and single cellular spore phases. The majority of fungal growth, however, occurs by mitotic division of the 1n haploid state. Specific fungal species can be saprophytic (degrade soil organic matter and dead plant materials) or pathogenic on plants (cause plant diseases). Some of the most devastating plant diseases are caused by fungi such as rusts of cereals and grasses, and the honey fungus causing white rots of many deciduous and coniferous trees. The width of fungal hyphae means they can occupy soil pores 5 micrometers in diameter and greater.
Water moulds
Water moulds are also called oomycotes and are filamentous (hyphal forming) multicellular eukaryotes more related to the giant kelp of the oceans than fungi! They are called water moulds because they prefer living in water and in water-saturated soil. As they have hyphae, they are often confused with fungi. The normal body state is diploid (2n). They can reproduce asexually (without genetic combination) by mitosis to produce 2n motile zoospores that germinate into hyphae. Zoospores need water-filled pores to use their flagella for motility. Sexual reproduction occurs by genetic combination of a male and female 2n hyphal strand followed by meiosis to produce a 2n non-motile oospore. Oospores are very thick-walled and can rest in the soil for years and withstand the stress of drying and freezing. Under wet and resource-abundant conditions (e.g., high density of root tips of crop seedlings), oospores germinate to produce many motile 2n zoospores. Species of oomycetes can be saprophytic or plant pathogenic. Plant pathogens can be nasty, such as the late blight pathogen of potato that helped cause the Irish Potato Famine of 1845-1849, Aphanomyces root rot of pulses, and Sudden Oak Death that is devastating oaks in California. Similar to fungi, the width of the hyphae of water moulds means they can occupy soil pores 5 micrometers in diameter and greater.
Green algae
Algae are non-plant, photosynthetic organisms (or “photoautotrophic”; see Table 6.1) whose cells contain organelles (i.e., they are eukaryotes). They are very important primary producers in aquatic ecosystems (e.g., lakes), and in aquatic and marine habitats span from single cellular to large seaweeds. Unicellular algae are found in wet areas on the surface of some soils where they receive sunlight, but typically are in very low abundance, and generally, algae don’t represent an important input of newly fixed carbon through photosynthesis to terrestrial ecosystems relative to plants. Green algae reside on wet soil surfaces and surface water-filled pores greater than 50 micrometers in diameter.
An important exception to this is in lichens that can be found in soil biological crusts and colonizing rocks, bare soil surfaces, and standing and fallen woody plant parts. Lichens are interesting cases where two species join together to form an entirely new “species”. Lichens represent an important mutualistic symbiosis (i.e., different organisms living together) and have both a “photobiont” and a “mycobiont”. In about 90% of lichen “species”, the photobiont is an alga, while the mycobiont is always a fungus. In this mutualistic relationship, the photobiont uses sunlight to fix CO2 into organic matter (i.e., supplying food), while the fungal partner provides most of the shape and physical strength. The fungi in lichens (chemo-organo-heterotrophs; Table 6.1) also obtains water and essential nutrients from the environment (e.g., nitrogen, phosphorus) in the partnership, and provides the physical anchor the keep the lichen in place. In ~10% of lichens, the photobionts are photosynthetic bacteria called Cyanobacteria. Recently, some lichens have been identified as being a tripartite symbiosis between an alga, cyanobacterium, and fungus. Lichens have the ability to live in very harsh environments, and are important early colonizers of barren soils, and even rocks (Figure 6.4)! Lichens that contain a cyanobacterium also have the neat ability to biologicallly fix atmospheric nitrogen gas to ammonia, which is usable by the fungal partner and input of plant-available nitrogen to the soil. Thus, lichens serve many important ecological roles as colonizers providing cover in exposed and new soils such as Regosols (Chapter 8) preventing erosion, building soil through the input of organic matter and plant-available nitrogen to the soil, increasing chemical weathering of mineral surfaces by the production of organic acids, and initiating succession of plants on soils.

Protozoa
Protozoa are single cellular eukaryotic (they have a nucleus and often other organelles) heterotrophic (they eat organic matter; see Table 6.1) microbes that can move about in water (they are motile). Although ranging in size, a typical protozoan in soil might be ten times larger than a soil bacterium, but still microscopic. Broadly, protozoa are grouped by how they move; amoeba shift their cytoskeleton and pull themselves with pseudopods, ciliates like paramecium are covered in short hair-like cilia that bend in unison to propel the cell, and flagellates use one or multiple long whip-like flagella for motility. Although much remains to be learned about the diversity and roles of protozoa in soils, many feed on bacteria, and in some cases fungi, and thus can play important roles in structuring the microbial community through this predation and contribute to the mineralization of carbon (Chapter 3) and nutrients such as nitrogen and phosphorus (Chapter 7) from the bodies of grazed microorganisms or small particles of soil organic matter. Protozoa are typically found in larger numbers in the rhizosphere where both organic matter and bacteria are abundant compared to root-free areas of soils. The populations of protozoa can oscillate with those of bacteria in soil, where populations increase in response to the growth in bacteria such as after rewetting of soil or addition of animal manures to the soil. As swimmers by definition, protozoa typically reside in the films of waters on soil particles and in water-filled pore spaces, and numbers are low in drier soils. Protozoa reside in water-filled pores greater than 50 micrometers in diameter.
Nematodes
Nematodes are multicellular eukaryotic, nonsegmented roundworms. They constitute as much as 90% of the number of multicellular animals in soil, and their numbers often exceed several million per m2 of A horizon (Chapter 2) soil! They are not worms such as earthworms; those are segmented worms and a very different group of soil organisms. Nematodes vary with species from 0.5 mm to 5 mm in length. Nematodes are long and narrow with either tapered or rounded ends (head and tail). Compared to the organisms we have learned about so far, nematodes are very advanced in the degree of specialization of their body components, having a mouth and esophageal system for feeding, digestive tract, anus for the elimination of wastes, glands and openings for osmoregulation with soil water, male or female reproductive organs, openings and various chemo and tactile sensory structures, and nervous system and rudimentary brain. Nematodes move through water-filled or near water-filled soil pores greater than 50 micrometers in diameter.
Can You Dig It!
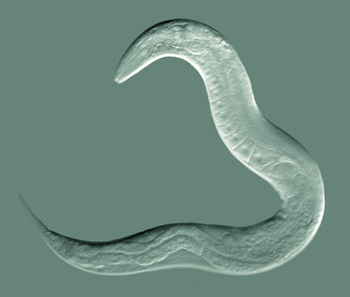
Nematodes are very hardy and fascinating animals. The most famous nematode is Caenorhabditis elegans, which is thought to have been first collected from a stockpile of animal manure in England. This nematode is a bacterial feeder and present in humid temperature soils with a plentiful supply of plant residues. Most C. elegans individuals are hermaphroditic worms, self-fertile females. It can be dried or frozen for decades and still be revived. It has been to space on many missions by NASA, was the first multicellular organism to have its genome sequenced, its cell development from egg to adult fully characterized, and first and only organism to have its nerve cells and nervous system completely mapped out. C. elegans, as scientists call the nematode, has been the subject of many breakthroughs in recent science history, including research leading to three Nobel Prizes, 2002 and 2006 in Physiology or Medicine, and 2008 in Chemistry.
A nematode species can be assigned to a specific trophic group being plant, bacterial, fungal, algal, omnivorous, or predatory feeding (Figure 6.5). Plant feeders are herbivores, and some can cause very damaging diseases such as the soybean cyst nematode, root-knot of tomato and potato early dying, but most plant-feeding nematodes do limited damage to plants. Omnivorous nematodes can feed on fungal hyphae, fine roots and hairs, other nematodes and mesofauna. Predatory nematodes feed on nematodes and mesofauna.
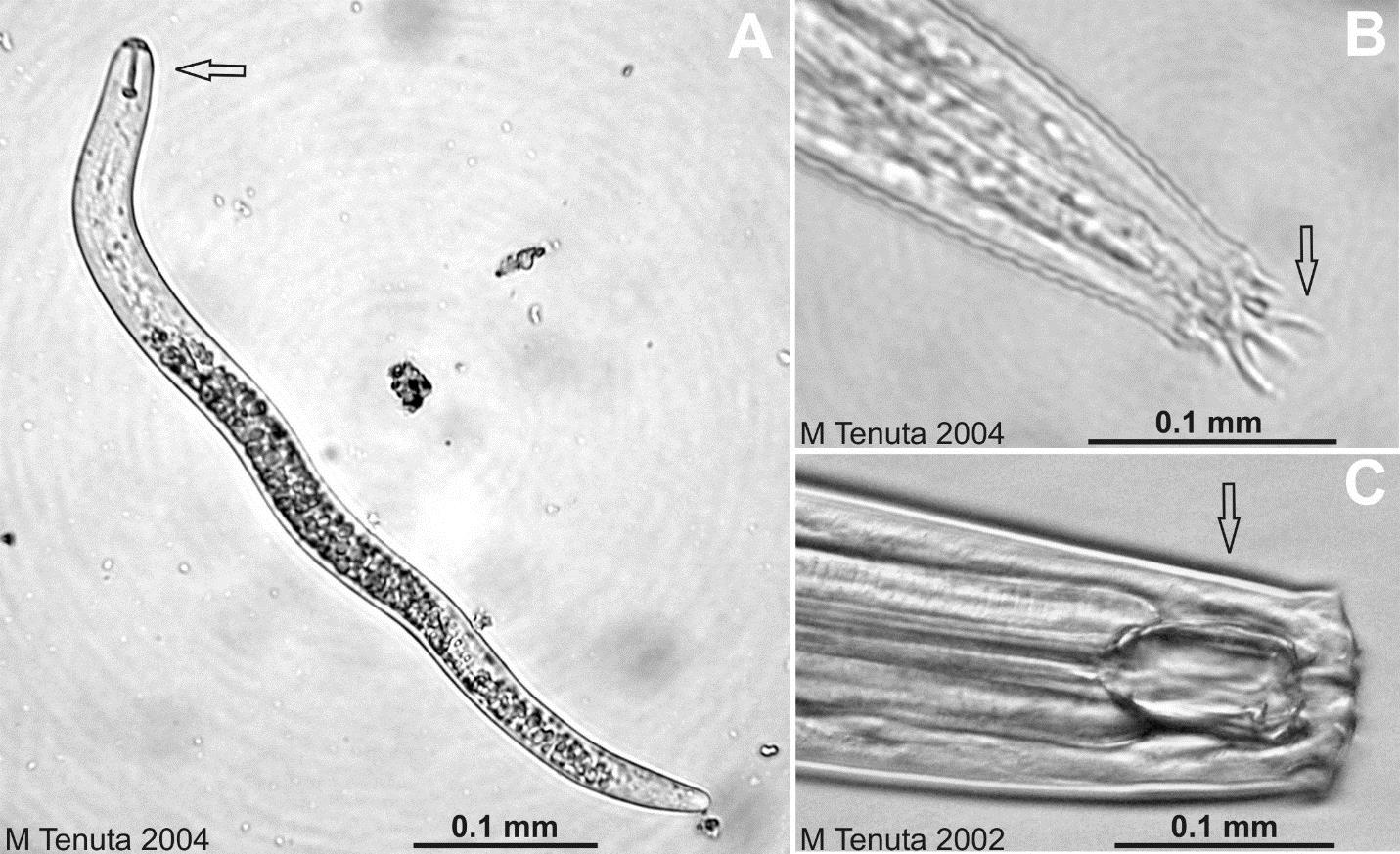
The ecological roles of nematodes involve introducing plant fixed carbon to the soil food web, increasing mineralization, particularly that for nitrogen from their food sources, regulating population and community diversity of food webs, and reducing levels of plant pathogenic and plant damaging nematodes through predation.
Nematodes are excellent bioindicators of the effect of management practices on soil health as the presence and population of individual species respond to populations of food sources in the soil food web with some species of nematodes being sensitive to soil management practices (e.g., tillage, fertilizers, pesticides, moisture, rotations, etc.) and stresses (e.g., contaminants such as metals). Many nematodes are parasites of plants. Some nematodes are r-strategists that respond quickly in population to levels of favourable food resources (e.g., bacteria) and soil conditions (e.g., moisture and temperature). Others are K-strategists that thrive in soils with little stress and fluctuating conditions; these are slow-growing, have long life spans for a nematode (one to many years). Then there are nematodes intermediate to r- and K-strategists that are usually most abundant in soil.
Microarthropods
Arthropods have exoskeletons, paired legs, and jointed appendages (e.g., think of insects and spiders). In soils, microarthropods are often dominated by two groups: springtails (of the sub-class Collembola, which is often used as a scientific name) and oribatid mites, or Oribadita (Figure 6.6). Collembola and Oribatida fall in the body-size category of soil “mesofauna” (see Figure 6.1), ranging from ~0.1 to 2mm in size, and similar to all of the groups of biota described above, are found in soils globally. Typically between 10 and 100 unique species of microarthropods exist throughout the profile of one square meter of soil and on decomposing vegetation aboveground, and microarthropod communities can contain up to 10,000 individuals throughout the profile of a one-m2 of soil. Soil microarthropods perform important functions through their feeding activities as fungivores (grazing on fungi), herbivores (e.g., grazing on small plant roots), decomposers (grazing on soil organic matter and plant litters), and predators (eating other microarthropod). Similar to protozoa and nematodes, some species of soil microarthropods have highly specialized feeding behaviours (e.g., specialists grazing on very specific types of fungal hyphae), while many of the herbivores and decomposers are generalists. Soil microarthropods can selectively influence the structure of the biological community (e.g., by selectively feeding on certain fungi or other arthropods), distribute microbial cells through the soil profile, and their fecal pellets can promote the formation of aggregates, which together with burrowing activities, improves soil structure (see Chapter 4). Microarthropods can reproduce sexually or asexually, and Collembola are typically thought of as early colonizers of recently disturbed soils (r-strategist) while Oribatida tend to colonize and reproduce slower and maximize numbers in older-growth ecosystems (K- strategist).

Other invertebrates
Soils and associated plant litters are also home to a very large diversity of other larger arthropods (ants, beetles, and others) with many generalist and specialist detritivores, fungivores, herbivores, and even carnivores (e.g., centipedes; scientific name Myriapoda). Annelids (worms; scientific name Oligochaeta) are also important macroscopic invertebrates found in soils, including a variety of earthworms. Earthworms are often revered by farmers and gardeners as being both indicators of healthy soils and contributors to soil health, but these sorts of earthworm paradigms don’t tell the full story (Figure 6.7). On the one hand, earthworms, notably the nightcrawler (Lumbricus terrestris) create deep borrows and casts that can improve soil structure, increase pore spaces in compacted soils, and speed up nutrient turnover from plant litters. In intensively managed ecosystems with repeated tillage, harvesting and organic residue management (e.g., crop stover, livestock manure), earthworms arguably do help. However, in unmanaged or less intensively managed ecosystems like long-harvest-rotation temperate and boreal forests, earthworms do more harm than good. There are very few native earthworm species in Canada; the exceptions are on Vancouver Island and in Yukon. The reason is that Canada was nearly entirely glaciated (97%) during the last glacial maximum of the Pleistocene Epoch (ending ~12k years ago), and earthworms have likely only become widespread following the proliferation of resource exploitation by European settlers that involved the movement of soil on tillage equipment and improper management of worms as fishing bait. Particularly in forest ecosystems, earthworm invasion- particularly by L. terrestris changes the structure of the soil profile, including largely eliminating organic horizons that are important habitat for plant seedlings, fungal networks, and fauna, including certain birds that nest in the forest floor. Additionally, nutrient retention in mature forest ecosystems is particularly high; long-lived trees producing mainly woody biomass need only small amounts of nutrients like nitrogen and phosphorus to grow each year, and correspondingly, the release of nutrients through decomposition in soils occurs slowly (see Chapter 7). Earthworm invasion leads to very rapid stimulation of microbial decomposer activity and the release of a finite pool of nutrients at a rate too fast for trees to use. It is no surprise that earthworms like L. terrestris have earned the title “ecosystem engineer”, alongside the likes of beavers, given the severity of the changes they facilitate to soils. For example, Charles Darwin estimated earthworms in a meadowed grass soil in England transported 5 cm of soil from below the surface soil to the surface over ten years!

Vertebrates
The biomass of vertebrates in soils is quite low compared to smaller biota, however, they can have an important influence in ecosystem functioning by way of altering soil physical and chemical properties (via tunnelling, mixing soils, and their excrement) and by selectively or indiscriminately feeding on other soil biota. A range of mammals (gophers, prairie dogs, groundhogs, etc.), amphibians (e.g., salamanders), and reptiles (skinks, lizards, turtles, etc.) spend at least portions of their lifecycles in burrows or under litter in the surface of soils. Even bird species like the ovenbird spend a portion of their life nesting in the forest soils of Central Canada and the North Central USA. Some vertebrates like the redback salamander are useful indicators of physical (tillage, forestry-related activities) and chemical (e.g., pollution) disturbance because they are reliant on stable habitat and food supply in the form of a robust arthropod community, and because they breathe through their skin.
Viruses
Viruses are smaller than even bacteria (0.05 to 0.2 micrometers), are not organisms and not “alive” in the accepted meaning of the term. However, despite being non-cellular entities, they are composed of biomolecules and play immense roles in disease and genetics in most, if not all, organisms and ecosystems. The simplest viruses are made up solely of genetic material (DNA or RNA) and a protein shell called a capsid. Virus particles are entirely dependent on their hosts- they “hijack” the host’s genome with their genetic code and instruct the host’s metabolic machinery (i.e., ribosomes for protein synthesis and DNA or RNA synthases for nucleic acid synthesis) to build 10s to 100s of new virus particles. This inevitably leads to cell death, and in the case of single cellular organisms like bacteria or protozoa, kills the host. Viruses that infect microorganisms are also called phages and broadly grouped based on the type of organism they infect (e.g., bacteriophage for bacteria, mycophage for fungi). Some viruses are vectored (transmitted) by soil organisms. For example, some plant-feeding nematodes harbour plant viruses in their esophagus that infect plants with feeding by the nematode. Little is known about the ecological importance of viruses in soil. However, because viruses are often specific to a species of an organism, they may be more diverse than any group of organisms.
Soils can serve as a reservoir for plant and animal viruses that cause disease; however, the role of viruses in ecosystem functioning is much less clear. The number of virus particles per gram of surface horizon soil can be as high as one billion. This is like concentrations seen in surface waters of aquatic ecosystems- and in the past two decades, virus roles in aquatic ecosystem functioning have become well understood. By lysing phytoplankton (cyanobacteria and unicellular algae, the primary producers in aquatic ecosystems) and heterotrophic bacteria, viruses are responsible for shaping biotic communities, and for releasing as much as one-quarter of all carbon fixed into biomass from CO2 during photosynthesis. Soil viruses may play similar roles, but with much higher biological richness (the number of unique species) and much more species evenness (i.e., without one or a few dominant taxa) in soils than waters, soil viruses will very likely have their own nuances in how they shape biological communities and ecosystem functioning.
Plant roots
Roots are soil biota, influencing soil development and properties as much as any group of organisms we have explored in this chapter. The biomass of roots is often higher than for all other soil organisms combined in soil that is vegetated. For example, a soil covered in perennial grasses can typically have 10 to 90 t ha-1, compared to fungi (2-5) and bacteria (1 to 2), and up to 2.5, 0.5, 0.2 t ha-1 for earthworms, protozoa and nematodes, respectively. The size of roots varies, meaning they occupy a variety of soil pore sizes, handy for obtaining nutrients and water in the soil. Root hairs are about 10 micrometers in diameter, fine roots up to 1 mm, and coarse roots above 1 mm in diameter.
Plant roots are very important in the ecology of soils. They are a major source of organic carbon materials and hence energy for soil heterotrophs and saprotrophs, they lower soil moisture and nutrients levels, consume oxygen through respiration, and alter soil chemical (e.g., soil pH) and physical (e.g., promote aggregation and macropore formation) properties. For more information on soil chemical and physical properties, see Chapter 3, Chapter 4, and Chapter 5.
Distribution of soil biota
Where does the bulk of soil biota reside within soil profiles? The simplest strategy for answering is to “follow the carbon”. Because most of the non-plant soil community are heterotrophic (see Table 6.1), and reliant on organic matter for both carbon and a source of electrons for generating ATP/energy, numbers of most of the types of soil biota introduced above are highest near the surface of soils and near plant roots in the rhizosphere and Ah horizon of soil (Figure 6.8 ). Even lithoautotrophic organisms (Table 6.1) tend to cluster around the organic matter inputs and other community members, as they are still dependent on the soil for many of their non-carbon nutrients (e.g., reduced mineral nutrients such as ammonium [NH4+] or ammonia [NH3] for nitrifying bacteria) for growth and metabolism. That said, even in lower abundance, microbes and other heterotrophs alike are still found throughout soil profiles if there are resources available. The importance of microbial biofilms in and around primary soil particles and peds is also worth mentioning. Biofilms form when even distantly related microbes signal to each other to produce sticky extracellular compounds that provide stable habitat (and trophic interactions within the biofilm), and thus allow microbial communities to build their own niches. Although motile soil microorganisms are important- including the protozoans that are defined as being motile, most soil bacteria are attached to soil particles or reside in biofilms.

What about the larger-scale distribution of soil organisms? For example, are the same species and communities found in similar soils on different continents? This is an intriguing question that, in some regards, is not perfectly answered yet. For larger organisms, in the absence of humans facilitating biological invasions by intentionally or unintentionally introducing species, the answer is probably “no”. Think of the nightcrawler example above- there are still “invasion fronts” of these earthworms in forests of Canada and the USA today following their introduction to North America post-colonization by settlers. What about for the very smallest organisms that tend to dominate the non-plant biological activity in soils- the bacteria, archaea, protozoa, and unicellular phases of fungi? Pioneering Dutch environmental microbiologists Martinus Beijerinck in the early 1900s and later Lourens Baas-Becking are credited with the hypothesis that “Everything is everywhere, but the environment selects”. In essence, there is a global ‘seeding’ of microorganisms given their small size (e.g., could be transported globally on small dust particles in the atmosphere), and they flourish when conditions and resources are right for them, regardless of where those conditions and resources are. Body-size-threshold paradigms have been proposed to explain (lack of) constraints on the natural distribution of biota (including soil biota). However, given that highly-mobile humans have touched nearly every soil environment on Earth, the role of introduced/exotic species is arguably equally as important.
How do we know who lives in the soil?
Studying what we cannot easily see- both because it is buried, but also because it is small- makes fields of soil biology/ecology both challenging and exciting. Analytical artifacts and biases are present in studies of any of the groups of soil biota we introduced above, but on most fronts, soil biologists are rapidly advancing the techniques used to study soil populations, communities, and dynamic interactions. It is surprisingly recently that we have discovered what we don’t know about the scope of soil biology. This perhaps began for soil bacteria with what is called “the great plate count anomaly”. Here, it was noted that the number of cells that could be extracted from soil and viewed/counted using a microscope was orders of magnitude greater than the number of colonies that could be grown on microbiological media in the lab from the same sample (Figure 6.9). The term anomaly was probably too harsh. Extracting and viewing cells from soil is relatively free from selective bias, however, trying to simulate soil conditions and resources in a synthetic medium in the lab is extremely challenging- it is no surprise that only a small fraction of the soil bacterial cells that could be viewed could be grown in the lab. Think back to what you have already learned in Chapter 3, Chapter 4, and Chapter 5 in this book. The chemical, physical, and ecological diversity and complexity in soils are immense at very small scales (i.e., humus molecules with molecular weights in the tens of kg per mole range; a single gram of clay with more surface area than a soccer pitch!). This small-scale complexity has given rise to virtually limitless biological niches that soil microbes and other organisms have evolved to exploit. Just within the bacterial realm a gram of surface soil in a productive ecosystem contains 10s of billions of individual cells from 10s to 100s of thousands of unique taxa. Likewise, the amount of biomass of soil biota is also staggering. A cultivated field can typically have 20 t ha-1 of roots, which is about the weight of 25 cows and 15 t ha-1 of other soil organisms being the weight of 20 cows! That alone is an overwhelming prospect, and this single section in a single chapter of an introduction to soil science textbook could comprise multiple textbooks on its own. Below we touch on just a few examples of both the challenges and excitement of methods for answering questions on soil biodiversity and ecology.

In-situ observations
Depending on the target population or community, different methods exist for studying soil biota in-situ. The soil “contact slide” approach involves inserting a glass slide into soil for a number of days to weeks and then observing attached microorganisms with microscopy. Invertebrates can be observed with pitfall traps, which are buried smooth-surface containers that are open on the top and allow soil fauna to fall in, but not climb out. Often times pitfall traps are partially filled with a preservative like ethanol to kill and prevent decomposition of the trapped fauna. (Find out how to make your own pitfall trap in the You Can Dig It! Activity #1). A particularly interesting and simple technique is commonly used to measure earthworms in soils: mustard powder (the same you would find in the spice section of your local grocery store) is mixed in a weak solution and poured over a defined area of soil. The mustard irritates the worms and they flee their borrows to the surface temporarily, allowing them to be counted, weighed, and identified to species, and there appears to be no long-term negative physiological effects from this contact (see You Can Dig It! Activity #2). Plant roots can be observed and tracked in-situ using clear tubes inserted in soils and a “rhizotron” camera that is inserted periodically in each tube. Image analysis software helps in identifying the same roots over time and quantifying their growth and death. Finally, for groups of soil biota that perform a specific task (often called “guilds”), rates of activities can be measured by quantifying changes in either reactants or products over time. For example, in upland soils, aerobic methane-oxidizing bacteria consume the trace atmospheric gas methane, an important global greenhouse gas even though its concentration in air is only approximately 0.00002%. Placing a chamber over a defined area of soil and measuring the decrease in methane trapped in the chamber over time gives a reasonable estimate of how active the methane-oxidizing bacteria are in the soil under field conditions.
Isolation approaches
A common approach in soil biology has been to isolate species from their soil environment and community so they can be studied directly (Figure 6.10). For microorganisms, the goal is often to obtain a pure culture (i.e., a population growing on an artificial medium in the lab) where physiological traits can be measured, including what resources are used, what products produced, and how changing conditions like temperature influence growth rates and survival. Much has been learned by cultivating microbes, but the approach is highly selective, and selecting one species from a community of thousands to millions is challenging. In the past few decades, biomarker fingerprinting approaches described below for characterizing soil microbes- particularly those using DNA sequences to identify specific taxa- have helped guide isolation and cultivation approaches towards environmentally relevant organisms and ignore “weeds” that inherently grow well under simple laboratory conditions.

Isolation of soil invertebrates differs from the isolation of microorganisms in that individuals are large enough that they can be observed and characterized on their own with microscopy (i.e., growing populations on artificial media is not needed to study them). Nematodes can be extracted with a Baermann Funnel system, where the soil is placed on a cloth mesh in the funnel and saturated. Nematodes are washed downward and through the cloth, while soil particles remain. A common approach for isolating collembolans and mites (groups of soil “mesofauna” see Figure 6.1) is the use of a Tulgren (sometimes also called Berlesse) Funnel extraction system. Here a light above a funnel filled with soil is a repellent for small soil fauna and they move downward until they drop through the stem of the funnel and into a container with a liquid preservative like ethanol below. Although individual specimens can be obtained with these approaches, taxonomic identification is still a major challenge and requires a great deal of time and expertise.
Biomarker approaches
Soil biota contains different unique biomolecules that can be extracted and characterized chemically to help study community composition, the relative size of a particular population or functional guild of organisms (e.g., nitrogen-fixing bacteria, see Chapter 7), or metabolic functioning of a soil community. These different biomolecules include cell membrane lipids that vary across broad groups of biota (for example, Archaea have a unique ether-lipid structure of their cytoplasmic membranes), sugar polymers that makeup cell walls (e.g., cellulose in plants and algae versus a compound called chitin in fungi and some invertebrates or a compound called peptidoglycan in bacteria), and very importantly nucleic acids (DNA and RNA). DNA can be extracted relatively easily from soil biota by pulverizing a soil matrix and binding the negatively charged nucleic acid mixture to a silica powder with high cation exchange capacity where it can be washed and then re-dissolved in pure water. Often taxonomic “marker genes” are targeted for sequencing and quantification (Figures 6.11 and 6.12). These are genes or other regions of an organism’s genome that are present in many or all organisms. For example, genes involved in producing a portion of the ribosome (protein synthesis factory present in every known organism) are commonly used to show similarity or dissimilarity in bacteria and archaea (16S rRNA gene), and fungi (18S rRNA gene or Internal Transcribed Spacer (ITS)), while a gene encoding for an oxidase enzyme involved in cellular respiration (Cytochrome Oxidase 1 (CO1) gene) is often used to show the relatedness of animal taxa. It is assumed that a more similar gene sequence between organisms means that they are evolutionarily more closely related. Techniques including the polymerase chain reaction (PCR) and high throughput DNA sequencing have become very accessible in research laboratories, and community fingerprints based on the DNA sequences of millions of individuals in a soil sample (i.e., how many of which individual taxa are in the soil) can be generated relatively cheaply and quickly (Figure 6.11 and 6.12). There are other nucleic acid based approaches, for example using quantitative PCR (qPCR) for estimating the number of copies of a particular gene in an original soil DNA extraction- and here genes could be associated with very specific functional guilds, for example like encoding for a portion of the nitrogenase enzyme complex involved in nitrogen fixation. Gene transcripts (i.e., messenger RNA or mRNA) can also be quantified with a variation of qPCR to characterize gene expression related to particular biochemical function. “Shotgun” sequencing of DNA and RNA extracted from soils is also a very powerful tool for characterizing the community and biochemical potential and functioning of soil communities. Referred to as metagenomic or environmental genomic sequencing, random fragments of nucleic acids are sequenced and analyzed, giving a picture of the overall community with less selective bias than PCR-based sequencing approaches.
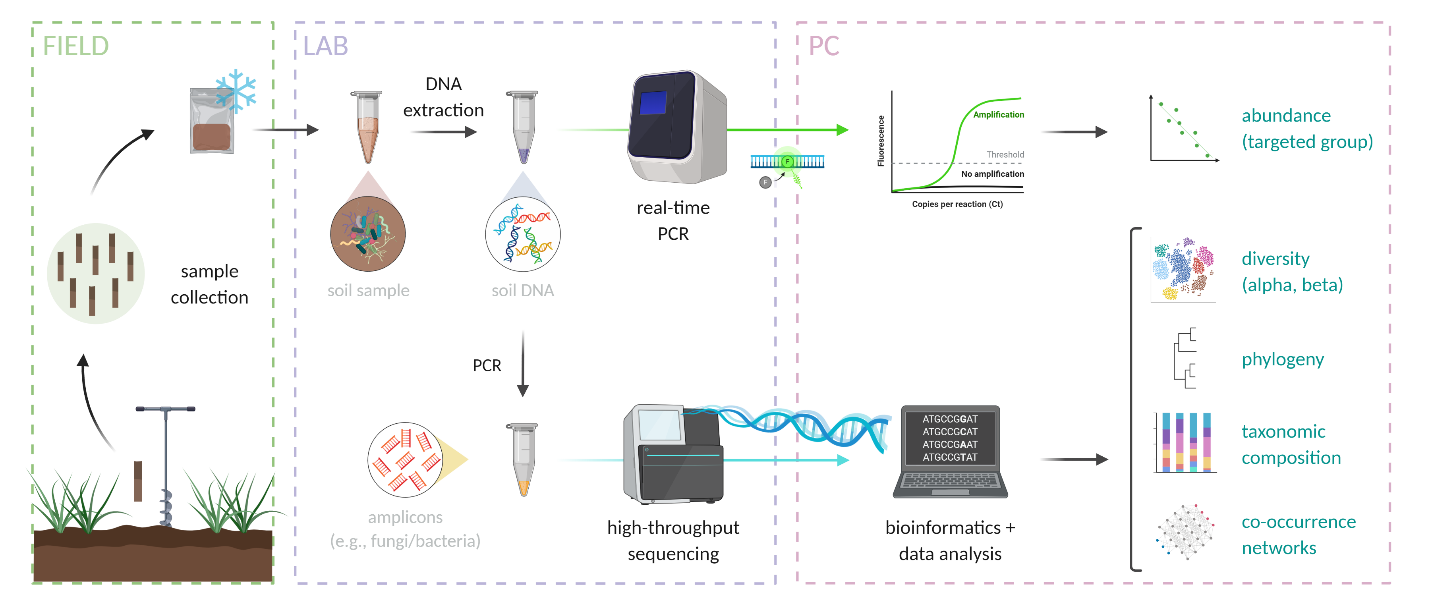

Although technologies for less selective nucleic acid extraction and quantification and inexpensive sequencing are ever-improving, a major challenge in the use of nucleic-acid sequence-based analyses of soil organisms and communities lies in the fact that very few taxa of soil biota globally have been described in detail (e.g., through isolation and cultivation approaches). For example, there are only approximately 10k identified unique strains of bacteria in culture collections globally, while up to potentially billions of unique bacteria across global soils. Fingerprinting approaches have taught us much about the diversity and ecology of soil biota over the past few decades, but at least at the time this chapter is being written, they have also shown us that there are many “known unknowns” waiting to be discovered!
SOIL ECOLOGY
What do soil biota do?
Ecology describes the interactions within a biological community and the dynamic interplay with the abiotic environment. Soil biota influence the soil environment in many ways that are covered throughout this book. For example, in Chapter 3 you encountered the still somewhat mysterious production and breakdown of soil organic matter that intimately involves soil communities, in Chapter 4 the role of biota in the formation of soil structure was highlighted, and next in Chapter 7 you will learn how key nutrient elements are transformed in soils, in large part a result of biological processes. Here in the present chapter, we take the approach of illustrating soil biological activities from the perspective of the organisms and communities. For example, the soil processes of nitrification and denitrification—where nitrogen is converted from one mineral form to another—are exceptionally important in terms of nutrient availability and loss from soils, and have regional and global importance in terms of how they affect water quality and the composition of the atmosphere. But if we step back and evaluate the directive of the microorganisms involved in these processes, they are- much more earnestly- just harnessing redox gradients in the environment to generate energy, grow, reproduce and successfully stay dormant in between periods of resource availability and proper environmental conditions. Next, we briefly present a number of important soil and related ecosystem functions carried out by soil communities in terms of the biochemical goals of the underlying guilds of organisms driving the processes that have broader ecosystem consequences. This complements the ecosystem-scale perspectives covered in other chapters.
Decomposition, cycling carbon, and methane
With the exception of plant roots, soil biota are predominantly heterotrophic and rely on fixed organic matter as the source of C for building biomass (anabolism) and as a source of energy (see Table 6.1). The initial input of food (i.e., organic matter) in soils is predominantly from plant litters and root turnover, however, organic amendments like manure can also be important large sources in crop and pasture systems. As you learned in Chapter 3, the quality and quantity of different plant litter influence rates of decomposition and formation of soil humus, and an important fraction of soil organic matter appears to be microbial by-products made from processing organic matter, rather than partially broken-down plant structures. In well-aerated soils, simple, and nutrient-rich compounds like sugars, starches, nucleic acids, and polypeptides can easily be mineralized to CO2 for energy generation and incorporated in biomass. More complex polymers like cellulose and chitin and aromatic polymers like lignin take longer to be converted back to mineral forms. Carbon dioxide is an important atmospheric greenhouse gas, and the role of soils in the global climate system and as mediators of global climate change is determined in large part by the rate of net carbon inputs to plant biomass minus the rate of release of CO2 by heterotrophic respiration as plants subsequently die and decompose.
What happens to heterotrophs when soils become saturated and no longer contain O2 rich air in pore spaces? In many soils, this happens periodically after a rain or snowmelt event; however, wetland soils are defined by prolonged saturation. Decomposition rates are much slower without O2, and this is one of the factors that contribute to the formation of organic soils (see Organic Soil order in Chapter 8); essentially, rates of plant growth exceed rates of heterotrophic mineralization over long periods of time. In bogs and fens (i.e., peatlands, the predominant ecosystem types with organic soils), soil profiles consisting of partially decomposed wetland plants can extend many meters deep (Figure 6.13)! Without oxygen, heterotrophic soil biota must rely on other biochemical pathways besides aerobic respiration to generate energy. Obligate aerobes only carry out metabolism when O2 is available, while obligate anaerobes cannot function in the presence of O2. The term facultative anaerobe (or facultative aerobe) refers to an organism that can generate energy and grow under O2 and O2-free conditions. Fermentative organisms carry out glycolysis- generating 1 or 2 ATP molecules per glucose molecule consumed- and then fermentation reactions to regenerate the molecules needed for glycolysis to continue. Fermentations generate reduced compounds like alcohols, organic acids, and H2 gas as waste products, and typically yield 18 to 36 times less ATP than glucose can provide an aerobe. Anaerobic respiration pathways are used by certain anaerobic soil bacteria and archaea to process plant litters and soil organic matter. In these reactions, compounds other than O2 are used in cellular respiration as the terminal electron acceptors. Denitrification and sulfate reduction are important examples of anaerobic respiration; denitrification in soils is typically carried out by facultative anaerobes and can yield up to 1/2 of the ATP per unit substrate as aerobic respiration, while sulfate reduction is carried out by strict anaerobes and yields much less energy. Methanogenesis is an important terminal anaerobic decomposition step in wetland soils (See Figure 6.13). A group of strictly anaerobic archaea utilize the waste products from fermentative soil bacteria (notably acetic acid and H2) and produce methane (CH4). Although energy yields from methanogenesis are relatively small, methane is a globally important greenhouse gas that is much more potent than CO2 per molecule, and changing methane emissions from wetland soils represent an important soil role in biosphere feedbacks to global climate change. Groups of bacteria called methane-oxidizing bacteria or methanotrophs can consume methane and use it both as a source of carbon and energy. Indeed, methane is a good source of electrons for ATP generation (similarly, it is a good source of heat in natural gas!), but it has high activation energy, and most methane oxidation in soils occurs under aerobic conditions, with O2 used as an electron acceptor. In wetland soils, methanotrophs reside in surface soil horizons where they can obtain methane produced under anaerobic conditions from below and O2 from the atmosphere above; they can be an important “filter” that keeps a portion of methane from reaching the atmosphere and contributing to the global greenhouse effect. Interestingly in upland soils, particularly those not disturbed by agriculture, methanotrophs consume atmospheric methane, representing an important global sink for this greenhouse gas, consuming about 5-10% of all methane emitted annually.

Nutrient cycling
The same types of activities by heterotrophic soil biota that break down organic matter and mineralize carbon as CO2 are also responsible for the release of important mineral forms of nutrients like nitrogen (as ammonium) and phosphorus (as phosphate). Uptake of nutrients for anabolism by non-plant soil biota is often termed immobilization, as the resources are at least temporarily unavailable to support plant growth, whereas plant root uptake of nutrients is termed assimilation. Certain groups of prokaryotes are also responsible for transforming mineral forms of nutrients as well. This includes the very specialized, energy-consuming process of nitrogen fixation where N2 in the atmosphere is chemically reduced and converted into available N build into amino acids (see below and Chapter 7) and the energy-producing processes of oxidizing reduced mineral forms or reducing oxidized mineral forms of nutrients. In the case of nitrogen, this involves the chemolithotrophic (Table 6.1) process of nitrification where ammonium or ammonia is used as a source of electrons that are typically transferred to O2 for ATP generation (producing nitrite and nitrate), and the anaerobic heterotrophic process of denitrification where nitrate is used as an electron acceptor during organic matter decomposition. These processes influence the availability of different forms of mineral N explored more in Chapter 7, and ammonium oxidation (the first step in nitrification) releases H+ ions and lowers soil pH, a consideration for farmers when spreading ammonium-based fertilizer salts. Both processes of nitrification and denitrification are multi-step and mediated by multiple enzymes. The potent greenhouse gas nitrous oxide (N2O) is an intermediate product and reactant in both. Although this means that soils can be either sinks or sources for atmospheric nitrous oxide, denitrification-related losses of nitrous oxide from fertilized agricultural soils represents one of the most important sources of this gas that contributes to both climate change and degradation of the stratospheric (upper atmosphere) ozone layer. Similar to nitrogen, sulphur and iron in reduced or oxidized forms can be both sources of electrons for chemolithotrophic microorganisms or electron acceptors for anaerobic heterotrophic respiration.
Phosphorus is essential for growth, because it is found in many critical biomolecules including nucleic acids, phospholipids, and ATP, and is often considered the second most limiting nutrient for productivity after N. Low soil P can decrease plant yields, while excess soil P that enters freshwater bodies due to surface runoff can cause a bloom in algal populations called eutrophication. The P cycle is unique because phosphorus does not exist in a gaseous form. Inorganic P present in the soil solution as orthophosphate ions (H2PO4– and HPO42-) is the only available form for uptake, but is usually found in very low concentrations (around 1-10 μM), because it is very reactive, and binds to cations and clays. P is primarily recalcitrant in soils in inorganic (sorbed P, primary and secondary minerals) and organic (organic matter, microbial biomass, living biomass) forms. Microorganisms can aid in plant P uptake through several mechanisms:
- iincreasing root interactions with orthophosphates by extending existing root systems through symbiotic associations with mycorrhizal fungi or stimulating root growth through the release of hormones (phytostimulation) by plant growth promoting rhizobacteria (PGPR);
- competing with plants for available P in the soil solution, immobilizing it into microbial biomass, and then regulating supply of P through microbial biomass turnover, and;
- solubilizing inorganic P through a range of metabolic processes conducted by a wide range of bacteria and fungi. These mechanisms include proton pumps, or organic acids, which solubilize precipitated (solid) forms of P (e.g., Ca-phosphates), siderophores that chelate metal ions associated with complexed forms of soil P, and microbial enzymes such as phosphatases that can hydrolyse organic P or cellulolytic enzymes that mineralize organic matter (see Chapter 7 for an explanation of “P fixation” v available forms of soil P).
Microorganisms with these capabilities can be used to increase the availability of soil P to plants. A fungus, Penicillum bilaiae was first isolated by researchers at an Agriculture Canada Research Station in Lethbridge Alberta (Kucey, 1983) and was commercialized as the first phosphate inoculant in Canada, JumpStart™, registered under the Canadian Fertilizers Act, for use on wheat in 1990, and is now registered for most crops grown in Canada.
Pollutant transformation
Soil biota, particularly some bacteria and archaea, play important role in the cycling of metal and organic pollutants. Similarly to iron, certain metals like arsenic can serve as both electron donors and electron acceptors for certain prokaryotic microorganisms depending on the conditions and resources in soils (e.g., concentration of oxygen and available organic matter), and the bioavailability and toxicity of arsenic is altered as its oxidation state is changed through microbial redox processes. Unlike with iron, however, arsenic has no structural role in biomolecules and indeed causes oxidative stress to cells and is severely toxic to certain organ systems in complex organisms. Cadmium is an element that is often present in trace amounts in mined agricultural fertilizers and can accumulate over repeated fertilization to levels of concern for food and feed supply. Unlike with iron and arsenic, prokaryotic microorganisms in soils don’t commonly harness reduced or oxidized forms for redox and ATP generation. The redox states of other metals like copper and nickel can be altered by certain soil prokaryotes for both energy generation and for anabolic use or detoxification. Copper, for example, is needed in small amounts for important microbial enzymes related to hydrolysis of organic matter and oxidation of methane by methanotrophic bacteria, while a trace amount of nickel is required for methanogenic archaea to produce methane. Both nickel and copper can be quite toxic in even moderate concentrations though- think that copper sulfate is the key additive in antimicrobial “pressure-treated” lumber, for example. For metals that are trace nutrients, much remains to be learned about how biota walk the line between utilization of and protection from the same elements.
In wetland soils, certain anaerobic bacteria and some archaea influence to the toxicity of mercury (Hg). In its elemental form, mercury is semi-volatile and can be transported globally in warm air masses. Primarily from coal burning, inputs of mercury deposition have more than doubled in many soils. Luckily elemental mercury (like you would find in an old thermometer) is not very bioavailable to organisms in soils, however in wetland soils where there is abundant organic matter and sulfate (e.g., like the peatland in Figure 6.13), anaerobic sulfate-reducing bacteria transform inorganic mercury into a bioavailable organic form called methylmercury. Methylmercury travels from soil water to surface waters where it bioaccumulates in organisms and biomagnifies in aquatic food chains.
Metal pollutant elements like arsenic, cadmium, and mercury cannot be fundamentally created or destroyed by soil biota, however, their toxicity and bioavailability can be changed by soil bacteria by altering redox states, or incorporation into a simple organic molecule like methylmercury. Organic pollutants that are typically synthesized for industrial processes or formed as inadvertent by-products during high-temperature industrial processes, can be fundamentally destroyed. Human-synthesized organic compounds like classes of triazine herbicides introduced in the 1960s and widely used in agriculture today initially were not biologically altered by soil bacteria. However, by the mid-1990s heterotrophic bacteria were isolated that had the ability to grow on the herbicides and break them down to a non-toxic form! Rapid evolution, including through horizontal gene transfer in bacteria, plays an important role in the ability to catalyze the breakdown of human-synthesized pollutant compounds. Soil bacteria certainly can’t process all of our organic pollution. In the case of crude oil and other hydrocarbon spills in soil, bacterial enzymes exist to catalyze breakdown; however, they tend not to be very active because hydrocarbon fuel pollutants tend to have very little nitrogen and phosphorus in them that is also needed for bacterial growth.
Plant-microbe interactions
There are many important interactions between plants and microorganisms in soils. The term symbiosis refers to different organisms living together, and although it is sometimes used to imply a mutualistic relationship, symbioses can also involve commensal interactions where only one of the organisms benefits and the other is generally unharmed, and parasitic relationships where one organism is harmed and one benefits (e.g., see on pathogens and parasites below). The rhizosphere is an important zone were plants and microbes interact. Heterotrophic decomposers gain energy and carbon from plant root turnover and root exudates, and in turn, mineralize organic forms of nitrogen and phosphorus into inorganic plant-available forms. This is a broad case of generally mutualistic symbiosis, however, when decomposing low-nitrogen-content plant litters like woody tissues, the relationship can shift and microbes can actually immobilize soil nutrients for a period of time to grow on the high carbon, low nitrogen material, leading to plant nutrient deficiencies.
Other plant-microbe relationships are much more direct. Symbiotic nitrogen fixation involves plants “farming” nitrogen-fixing bacteria in root nodules- the bacteria are fed carbon compounds pulled from the plant’s Krebs cycle, and in turn, the bacteria use a portion of the energy to convert atmospheric N2 into a chemically-reduced bioavailable form for the plant that is pushed back into the Krebs cycle and subsequently amino acid synthesis, allowing the plant to produce high-N containing compounds like chlorophyll and rubisco (a key enzyme in photosynthesis). Only certain plants and microbes can form these mutualistic symbioses. Legumes pair with a group of bacteria called rhizobia, while members of some shrubs like alder and sweet gale pair with members of the bacterial phylum Actinobacteria. Nitrogen fixation is exceptionally costly- with up to 16 ATP consumed per single molecule of atmospheric N2 reduced, and thus symbiotic N fixation is a good adaptation- as plants can readily produce energy-rich compounds through photosynthesis, whereas free-living heterotrophic soil N fixing bacteria cannot. The rates of N fixation between free-living and symbiotic root-associated bacteria are astoundingly different, with input rates of available N to soil jumping from 1 kg N ha-1 year-1 up to 200 kg!
Another important plant root-microbe symbiosis that was introduced above is mycorrhizae (see Figure 6.3). Many plant species allow colonization of roots by mycorrhizal fungi and supply the fungus with sugars, while the fungus helps the plant acquire nutrients like phosphorus, wards off plant parasites, and improves the soil structure in the rhizosphere. The symbiotic relationship in mycorrhizae is usually mutualistic, and recent research has shown that both plants and fungi have important mechanisms to choose partners who are going to facilitate the other in the relationship. In this regard, a fungus cannot utilize carbon-rich food provided by the plant without also doing its part and helping the plant acquire important limiting nutrients from soil like phosphorus.
Trophic interactions
In soil, the transfer of organic energy and nutrients in the form of molecules in the body of organisms determines the kinds and population levels of organisms in those communities. The concept of the soil food web is used to describe the community of organisms in soil for all or part of their lives. Food webs can depict the transfer of energy or nutrients between organisms within a community, but usually, it focuses on the flow of organic energy. These flows can be extremely complex, governed by the feeding needs of organisms. The steps in energy transfer (feeding of one organism on another) are referred to as trophic interactions.
The basis of most soil food webs is the production of organic energy by autotrophic organisms (largely photosynthesizing plants and referred to as primary producers). This energy is introduced to the soil through the dropping of dead above-ground parts (leaves, stems, trunks, crop residues) and roots. Organic energy and nutrients in dead plant parts and roots are used or released by decay organisms that are heterotrophic and called decomposers. Many species of bacteria and fungi are decomposers. From here on, the trophic interactions are done by heterotrophic organisms collectively called consumers.
Have you ever wondered how large dead plant parts disappear in time in soil? The feeding action of microarthropods that chew and shred those parts into smaller pieces is key. They are called detritivores and their main feeding interest is consuming the decomposers on dead plant parts. Their activity produces smaller dead plant parts, which in turn contains a greater surface area for continued colonization and decay by decomposers.
Living roots are also fed upon directly by a wide range of organisms including many bacteria and fungi, root-feeding nematodes, and microarthropods collectively referred to as herbivores and sometimes primary consumers. Herbivores can be detrimental or beneficial to the health of plants. Some bacteria and fungi, called plant-pathogens, target roots to feed by either killing root parts before feeding or feed on living cells, slowly draining roots of energy and leading to the eventual death of roots (Figure 6.14).

Soil fauna that kill parts of roots or compromise the health of plants are referred to as plant-pests (Figure 6.15). Many root-feeding nematodes and microarthropods are plant-pests. Beneficial herbivores obtain energy from roots and promote the health of those plants and thus called plant growth promoting rhizo-organisms (PGPR). They live in the rhizosphere (in the soil around or on and in roots) to obtain energy from plants. Examples of beneficial herbivores include biological nitrogen-fixing bacteria in the roots of legumes (e.g., bacteria of the order Rhizobiales in which the genera Bradyrhizobium forms nodules on soybean roots) and plants of the alder family (ex., bacteria in the genus Frankia: Figure 6.16). Examples of beneficial fungal herbivores are ecto- and arbuscular mycorrhizal fungi (Figure 6.3).


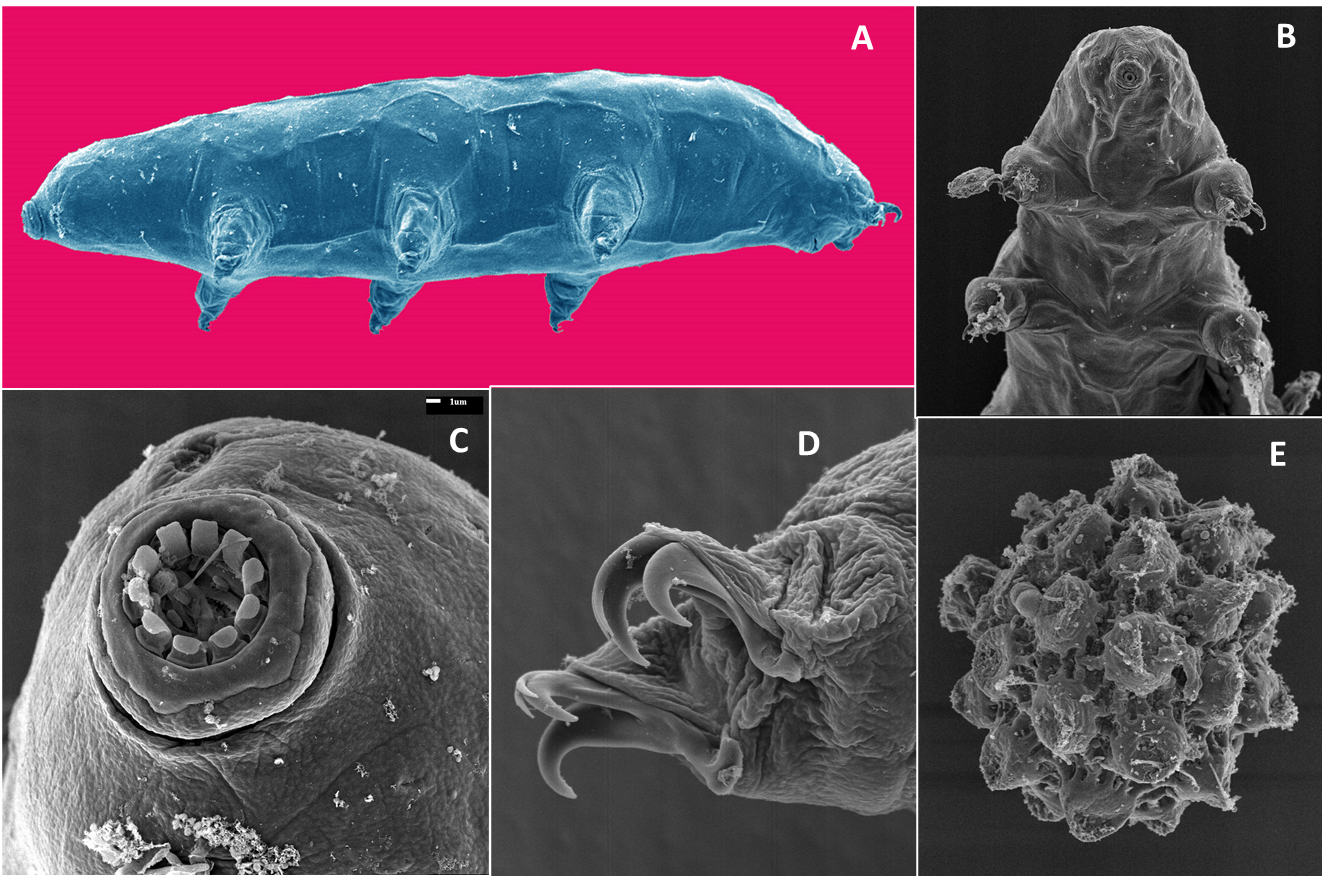
Numerous organisms feed on decomposers, plant-pathogens, and beneficial bacteria and fungi and are called microbial grazers or sometimes secondary consumers. They are considered carnivores as they do not feed directly on living or dead plants. Grazers often specialize to prey on bacteria or fungi, but rarely both. Feeding on bacterial grazers involves taking up the whole organism by the predator. Examples of grazers include amoeba, ciliate and flagellate protozoa, rotifers, bacterial feeding nematodes, and Collembola. Examples of fungal grazers include tardigrades (Figure 6.17) and fungal-feeding nematodes, mites, and Collembola.
Tertiary consumers feed upon microbial grazers and plant-pests. They can be fully carnivorous or omnivorous (feeding on combinations of fungi, soil animals and living roots and other plant parts. Examples of tertiary consumers include omnivorous nematodes, entomopathogenic nematodes (nematodes that kill and feed on microarthropods), nematode-trapping fungi (fungi that immobilize and feed on nematodes), and predatory nematodes and mites.
The trophic interactions of soil food webs can be described as diagrams showing trophic organisms or groups and the direction of energy flow. Soil food webs are analyzed by carefully sampling and processing soil and using methods for recovering and enumerating organisms present. The feeding preference of recovered organisms is either well documented as for those of economic importance such as pathogens and pests, or apparent—based on feeding structures of the organism. For example, predatory mites have a more massive piercing structure (chelicera) than fungal-feeding mites because of differences in prey size.
An example of a soil food web from the prairies of northeastern Colorado is shown in Figure 6.18. Organisms of similar trophic levels (preferred feed source) are given in boxes with the direction of food web energy flow in trophic interactions (food source/prey to consumer/predator) shown with arrows. The general trophic levels of primary producers, herbivores, decomposers, microbial grazers, and tertiary consumers are given. Note that the apex predator (top carnivore) in this food web are predaceous mites.

Primary producers and consumers in a food web have important roles in the soil, such as to balance populations of beneficial and detrimental organisms to plants. Food webs that are out of balance are prone not to promote the growth of plants. Balance in soil food web structure is controlled by bottom-up and top-down processes. Bottom-up processes involve the level of a resource determining the population of consumers in the soil food web. For example, where the levels of microbial and tertiary consumers are related to the density of roots in the soil, bottom-up control is evident (i.e., level of energy input via roots determines populations in higher trophic interactions). Top-down processes are where the levels of the predator determine that of the prey. In agriculture and forestry, top-down control of pest organisms is applied in biological control practices. Here the health of a plant affected by an herbivore is improved by the addition of predator of the later. Examples of biological control agents include entomopathogenic nematodes that kill lawn grubs or predatory mites to control populations of gnats and thrips.
Soil food webs conform to the ecological pyramid principle. Soil food webs usually have much higher amounts of energy in the biomass of primary producers (i.e. the plants), and the amount of energy/biomass decreases sequentially in decomposers, microbial grazers and finally tertiary consumers, whose overall biomass is smallest in a given area of soil. This observation is strong evidence that bottom-up control of soil food webs is important. Figure 6.18 shows that for a typical grassland plant, roots have the largest biomass of all soil organisms, followed by fungi and bacteria (presumably largely decomposers) then microbial grazers. When present in soils, earthworms, feeding on anything in ingested soil, likely derive most of their energy from detritus, bacteria and fungi.
The same reasons shaping the ecological pyramid above-ground is in effect in soil. Each trophic interaction (feeding activity) results in loss of organic energy as heat. This means there is less energy transferred up the food web. Typically, we don’t think of organisms in soil as producing heat as mammals do. But if you have ever composted, you know temperatures can get as high as 65°C at the centre of large piles. Compost is an excellent insulator for heat, thus retaining heat produced by decomposers (Figure 6.19). Other reasons for an ecological pyramid are predators expend energy seeking prey and predators can live longer than prey.

SUMMARY
- The very large diversity of chemical, physical, and ecological niches in soils have selected for diverse biota spanning from micrometer-sized bacteria to vertebrate fauna; soil biota dynamically responds to and shapes their soil environments.
- Small size, high numbers, and lacking databases and expertise in taxonomy represent important challenges to our understanding of the scope of diversity in soils- there are many new discoveries to come!
- Different metabolic strategies are used by soil organisms in terms of how they harness energy and nutrients from the environment; however, heterotrophic activity predominates.
- These metabolic processes represent biotic directives of generating ATP, surviving, and reproducing, but from the soil ecosystem perspective, the associated resource exploitation essential drives processes such as organic matter and nutrient cycling (influencing soil fertility), among many others.
- Soil food webs depict trophic interactions between organisms and the flow of organic energy through biota; trophic levels in soil are primary producers, herbivores, decomposers, microbial grazers, and tertiary consumers; and bottom-up control where the amount of organic energy input to soil from plants generally controls the structure (biomass) of soil trophic levels.
SUGGESTED READING
Coleman, D., Callaham, M. and Crossley, D. 2017. Fundamentals of Soil Ecology. 3rd Ed. Academic Press, London, UK.
De Wit, R. and Bouvier, T. 2006. ‘Everything is everywhere, but, the environment selects’; what did Baas Becking and Beijerinck really say? Environ. Microbiol. 8: 755-758.
Hunt, H.W., Coleman, D.C., Ingham, E.R., Ingham, R.E., Elliott, E.T., Moore, J.C., Rose, S.L., Reid, C.P.P., and Morley, C.R. 1987. The detrital food web in a shortgrass prairie. Biol. Fertil. Soils 3:57–68.
Kingston, W. 2004. Streptomycin, Schatz v. Waksman, and the Balance of Credit for Discovery. Journal of the History of Medicine and Allied Sciences 59:441-462.
Kucey, R. M. N. 1983. Phosphate-solubilizing bacteria and fungi in various cultivated and virgin Alberta soils. Can. J. Soil Sci. 63:671_678
Madsen, E.L. 2015. Environmental Microbiology: From Genomes to Biogeochemistry. 2nd Edition. Wiley-Blackwell, Hobokon, NJ.
Nair, P. 2012. Woese and Fox: Life, rearranged. P.N.A.S 109: 1019–1021.
Paul, E.A. 2015. Soil Microbiology, Ecology and Biochemistry, 4th Edition. Academic Press, Boston, MA.
Richardson, AE., and Simpson, R.,J. 2011. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 156: 989-996. DOI: 10.1104/pp.111.175448
Schmitt, C.L., and Tatum, M.L. 2008. The Malheur National Forest:Location of the World’s Largest Living Organism [The Humongous Fungus]. United States Department of Agriculture- Forest Service. Available from http://www.fs.usda.gov/Internet/FSE_DOCUMENTS/fsbdev3_033146.pdf [accessed 26 October 2020].
Simard, S., Perry, D., Jones, M., Myrold, D.D., Durall, D.M. and Molina, R. 1997. Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 388:579–582
Swift, M.J., Health, O.W., Anderson, J.M. 1979. Decomposition in Terrestrial Ecosystems. University of California Press, Berkley, CA.
STUDY QUESTIONS
1) Why is the diversity of soil bacteria so high? What roles do soil properties you learned about earlier in the text and trophic interactions among soil biota play in this high level of diversity?
2) What are biomarker and isolation based approaches to studying soil biological communities? Why are biomarker approaches particularly useful for characterizing the diversity of small-sized biota in soils?
3) Give one example each and describe the nature of the relationship in a mutualistic and a parasitic symbiotic relationship between soil organisms.
4) Where is biomass typically largest in the soil profile and why? How does this relate to the dominant metabolic strategy (e.g., heterotrophy, lithotrophy, autotrophy) in belowground ecosystems?
5) Imagine a sterile soil entirely devoid of biota. Could this soil support plant group? Why or why not?
6) Describe differences in soil biota in a flooded (anoxic) wetland soil relative to a well-drained upland soil.
7) How does soil biota influence the cycling of carbon in both soils and the atmosphere?
8) Draw a hypothetical soil food web.
Can You Dig It!
Activities
Activity 1: Make a Pitfall trap to catch Soil Invertebrates and Insects
Adapted from: https://www.nhm.ac.uk/discover/how-to-make-pitfall-trap-to-catch-insects.html
You will need:
- a hand trowel
- a small plastic container or plastic cup
- a tray
- ID guides (optional)
- Choose a location for your trap on flat ground near vegetation.
- Use a trowel to dig a small hole.
- Place a clean plastic container in the hole. Fill in any empty space around the container with soil. Make sure that the top of the pot is level with the ground, or you won’t catch anything.
- Leave your trap overnight.
- Empty the trap into a tray to see what creatures wandered in. Use ID guides or apps such as iNaturalist supported by the Canadian Wildlife Federation and the Royal Ontario Museum Biodiversity Program https://inaturalist.ca/) to help you identify what kind of invertebrates they are.
- Record your findings: take pictures, make a note of what you caught, the date and location.
- Carefully release the creatures, returning them to a safe, sheltered place.
- Return the area back to how you found it.
Activity 2: Counting Earthworms
Adapted from: Christina Curell, Michigan State University Extension – June 29, 2016
https://www.canr.msu.edu/news/earthworms_can_be_an_indicator_of_soil_health
Materials that you will need to measure worms in the field:
- Tape measure
- 2 liters of tap water
- Hand trowel or shovel
- Container to collect worms
- Solution of 2 tablespoons of mustard powder dissolved in 2 liters of water
Once you have your materials gathered you are ready to count earthworms.
Step 1: Measure a square foot in the test area and dig down 12-inches.
Step 2: Collect and count the number of worms found. If possible, differentiate worms by type. For example, label as earthworms, red worms, etc.
Step 3: (Optional) Level out the bottom of the hole, and pour the mustard solution slowly. Deep burrowing worms should come to the surface within 5 minutes. Collect and count the worms that come to the surface.
Step 4: Count and record the total number of earthworms that are collected.
About the authors:
Nathan Basiliko, Professor, Faculty of Natural Resources Management, Lakehead University

My research group focuses nearly entirely on soil biology and the roles in biogeochemical cycles. However, because my academic “home” for the past 7 years at Laurentian University has been in our Biology department, I don’t teach soil science courses anymore (I used to). I miss teaching introductory soil science in particular, but my current position does give me a new opportunity to squarely build soil science in to other courses- including introductory and advanced microbiology and ecosystem ecology courses. I hope that this has some broader impact by engaging students with soil science who otherwise would not take soil science courses.
Kari Dunfield, Professor in Applied Soil Ecology and Canada Research Chair in Environmental Microbiology of Agro-Ecosystems, School of Environmental Sciences, University of Guelph

Kari is a Professor in Applied Soil Ecology and Canada Research Chair in Environmental Microbiology of Agro-Ecosystems in the School of Environmental Sciences at the University of Guelph (Guelph, Ontario). Her research group works at the intersection of microbiology, ecology, and soil science, and takes an inter-disciplinary approach to find solutions to complex global environmental challenges, including, recycling nutrients and minimizing greenhouse gas emissions in agriculture systems; breaking down pollutants in brownfield sites; and understanding pathogen transport from agricultural systems into waterways. I love studying soil microbiology because it is a combination of field and lab based research. In my group you need to own a pair of steel toed boots and a lab coat, because in the morning you could be going out to the field, using soil probes and shovels to get soil and root samples, and in the afternoon you could be back in the lab doing delicate and sterile molecular biology techniques.
Mario Tenuta, Professor of Applied Soil Ecology and Senior Industrial Research Chair in 4R Nutrient Stewardship, Department of Soil Science, University of Manitoba

As a Soil Ecologist, the work is very diverse and full of new challenges and learning opportunities. My research involves tackling the issue of lowering the amount of greenhouse gases emitted from agricultural soils. For this we do field research to understand how microbes are involved in nutrient cycling, how soil conditions affect microbial activities and functions and how farmer practices of fertilizer and manure use affect microbes. There is lots of opportunity for laboratory analysis of microbes, nutrients in soil, nutrients in plants, and of crop productivity. We take that understanding to recommend practices farmers can use to lower emissions of important greenhouse gases such as nitrous oxide (N2O). Soil is everywhere and the work also provides lots of opportunity to travel locally and the world doing research, teaching and development
All the organisms that occupy a specific habitat and interact with one another in time and space.
(biology) All individuals of a species living in defined geographical area at the same time in association with each other.
(ecology) A group of organisms that may interbreed and produce fertile offspring having similar structure, habits, and functions. Species ranks next below genus, as a fundamental unit in the hierarchy of classification. The name of the species becomes the second word of binomial nomenclature systems. Abbreviated as sp. (singular) and spp. (plural). As described in Chapter 6, the "species concept" is challenging to apply for many microorganisms, particularly the bacteria and archaea.
A functional unit consisting of all the living organisms (plants, animals, and microbes) in a given area, and all the non-living physical and chemical factors of their environment, linked together through nutrient cycling and energy flow.
Single cellular organisms that typically lack internal membrane-bound organelles, and notably, never contain nuclei. Members of the domains of life Bacteria and Archaea are prokaryotes.
A cell that has a membrane-bound nucleus, membrane-bound organelles, and chromosomes in which the DNA is associated with proteins; an organism composed of such cells.
An organism capable of utilizing light energy for growth; an organism that uses sunlight as its primary energy source for the synthesis of organic compounds. The word photoautotroph is a combination of autotroph, the word for an organism that makes its own food, and the prefix photo-, which means “light”.
An organism able to derive carbon and energy for growth and cell synthesis by utilizing organic compounds.
(1) The absence of molecular oxygen. (2) Growing in the absence of molecular oxygen (e.g., anaerobic bacteria). (3) Occurring in the absence of molecular oxygen (e.g., a biochemical process).
A type of bacterial or yeast metabolism (chemical reaction) characterized by the conversion of carbohydrates to acids or alcohols, usually occurring in the absence of molecular oxygen.
The fungal partners in mycorrhizae.
The association, usually symbiotic, of fungi with the roots of seed plants. Under favourable soil conditions, plants with mycorrhizae have been shown to produce up to four or five times as much growth as similar plants without mycorrhizae. The mycorrhizae absorb water and nutrients and pass them on to the plant, secrete hormones that stimulate plant growth, and help protect the plant roots from disease organisms. In return, the fungi depend on the plant for carbohydrates.
A mass of threadlike filaments, branched or composing a network, that constitutes the vegetative structure of a fungus. (plural mycelia)
The area of soil immediately surrounding plant roots in which the kinds, numbers, and activities of microorganisms differ from that of the bulk soil.
A mycorrhizal association in which the fungal mycelia extend inward, between root cortical cells, to form a network, and outward into the surrounding soil. Usually the fungal hyphae also form a covering or mantle on the surface of the roots.
Mycorrhizal association with intracellular penetration of the host root cortical cells by the fungus as well as outward extension into the surrounding soil. Also known as endomycorrhiza.
A cell or organism that contains a single set of chromosomes.
Pertaining to cells or organisms having two sets of chromosomes.
The process of nuclear division in a cell by which the chromosomes are reduced to half their original number; occurs during the formation of sex cells.
A flexible, whiplike appendage on cells, used as an organ of locomotion (plural flagella).
The edible seeds of any leguminous plant.
Organism resulting from the symbiotic relationship between a fungi and algae or cyanobacterium. They live in a wide range of habitats, from desert to polar regions and the tropics, but are commonly found in barren environments, and are dominant in tundra regions and mountains. Lichens are very sensitive to air pollution and serve as an indicator species. They occur in one of four basic growth forms: crustose- crustlike, growing tight against the substrate; squamulose- tightly clustered and slightly flattened pebble-like units; foliose- leaflike, with flat sheets of tissue not tightly bound; fruticose- free-standing branching tubes.
An interaction between two different organisms living in close physical association. Interactions may be mutualistic, commensal, or parasitic.
(r-selected species; from logistic growth curve equation referring to growth rate) Species that are characterized by having high growth rates, producing many offspring and flourish in resource-rich, less-crowded conditions that are unstable or fluctuating in environmental conditions.
(K-selected species; from logistic growth curve equation referring to maximum carrying capacity of the ecosystem) Species that are characterized by having few, slower slow-growing and long-lived offspring that flourish in stable environmental conditions.
A species that has a very selective feeding niche (e.g., that a nematode that only grazes on fungal hyphae).
A species that has very a broad feeding niche (e.g., a consumer in soil that will feed upon multiple types of living plant tissues or detritus as well as fungal hyphae).
Animal that feeds on particulate material derived from the remains of plants or animals, including large scavengers, smaller animals such as earthworms and some insects, and decomposers.
Any organism that derives its nutrition from dead or decayed organic material, in contrast to a parasitic organism that obtains its nutrition at the expense of a living organism.
An organic acid composed of purine and pyrimidine bases, sugars, and phosphoric acid joined into nucleotide complexes. Biopolymers present in all organisms responsible for genetic coding and storage, gene expression, protein synthesis, and even some catabolic activities. Nucleic acids encompass ribonucleic acid polymers (RNA) deoxyribonucleic acid polymers (DNA).
An in-vitro method for amplifying defined segments of DNA. PCR involves a repeated cycle of oligonucleotide hybridization and extension on single-stranded DNA templates.
The metabolic process involving the conversion of simpler substances to more complex substances or the storage of energy. More generally, the synthesis of organic compounds within an organism. Also called assimilation, biosynthesis, or constructive metabolism. See catabolism.
(biochemical) The conversion of an organic substance to an inorganic form, often as a result of microbial decomposition. Gross mineralization is the total amount converted, and net mineralization, the gross minus the growth demand or immobilization by the decomposer organisms, usually measured in laboratory incubations.
Organism requiring oxygen for growth.
(also facultative aerobe) The ability of certain organisms to harness energy from the environment under both aerobic and anaerobic conditions.
A metabolic process in which electrons are transferred from a reduced organic or inorganic compound to an inorganic acceptor molecule other than oxygen. The most common acceptors are carbonate, sulfate, and nitrate.
Biological production of the reduced simple hydrocarbon (and atmospheric greenhouse gas) methane (CH4); carried out by anaerobic members of the domain Archaea using a limited number of simple substrates that are produced by symbiotic fermentative bacteria.
The promotion of plant root growth facilitated by compounds released by rhizosphere microorganisms.
Rhizobacteria (bacteria from the rhizosphere and rhizoplane) that colonize plant roots and stimulate plant growth. They enhance plant growth directly (e.g., biological nitrogen fixation) or indirectly by protecting plants from disease and abiotic stresses such as drought.
Determined by microbial cell production and cell death.
The state of a compound being easily taken up by an organism, typically referring to an energy or anabolic nutrient source, or a pollutant or toxicant.
The process of a steady buildup of a contaminant in an organism over its lifetime (also see biomagnification).
The increase (often exponential) in tissue concentrations of contaminants up trophic levels in food webs to apex organisms.
The movement of genetic material by means other than parent to offspring.
Interaction between two species in which one species derives benefit while the other is neither benefited nor harmed.
Interactions between species where one benefits and one is harmed.
The interrelationship among the organisms in a soil community according to the transfer of useful energy from food resources to organisms eating those resources.
Processes of energy and nutrient transfer from one or more organisms to others in an ecosystem.
Autotrophic organisms in an ecosystem, i.e., those capable of reducing the carbon molecule in CO2 to form new organic carbon molecules such as sugar. Primary producer biomass serves as the energy and carbon input to an ecosystem, fuelling all subsequent consumption.
A heterotrophic organism, chiefly a microorganism (bacteria or fungi), that breaks down the bodies of dead animals or parts of dead plants and absorbs some of the decomposition products while releasing nutrients usable by producers.
An organism that gains energy by feeding on another organism. The place for a consumer in the food chain is defined by what it eats. Herbivores eat plants, and are primary consumers; a human can be a primary consumer by eating plants or a secondary consumer by eating an animal that feeds on plants.
A consumer that eats plants or other photosynthetic organisms to obtain its food and energy.
A microorganism, such as a bacterium or fungus, that has the capacity to cause disease under normal conditions.
Any organism regarded as harmful, irritating, or offensive to humans, either directly or indirectly through its effect on animals and plants; the term is typically applied to rats and other rodents, insects that transmit disease or destroy crops, and pathogenic fungi and bacteria. In agriculture and forestry, a pest is any agent designated as detrimental to the health of vegetation or animals and which impedes effective resource management.
Consumers that feed on decomposers or plant pathogens.
Species that feed upon microbial grazers and plant-pests. They can be fully carnivorous or omnivorous (feeding on combinations of fungi, soil animals and living roots and other plant parts).
Trophic interactions in a community, where the level of a primary resource determines the population of consumers.
Trophic interactions in a community, where the levels of the predator determine that of the prey.
A method of controlling pests or undesirable species by introducing or manipulating naturally occurring predatory organisms or their products, or by sterilizing them. In agriculture, biological control is used to reduce or replace mechanical or chemical means of controlling pests.
A visual representation, resembling a pyramid, that depicts the total mass of organisms residing in each trophic level in a given area. The bottom bar of the pyramid represents the mass of plants, the top bar represents the mass of carnivores, and intermediate bars represent other forms of biota in the feeding structure of the community.

