Iron Deficiency Anemia
Red cell breakdown products are not available for recycling when blood is lost from the body. Therefore, longstanding blood loss can result in a lack of iron for erythropoiesis. Chronic or recurrent external blood loss can be caused by intestinal hemorrhage from ulcers (drug or stress-induced), enteritis, or neoplasms; genitourinary bleeding; infestation with blood-sucking intestinal or ecto-parasites; and disorders of primary hemostasis, such as thrombocytopenia and von Willebrand disease. It is important to recognize that bleeding into the intestinal and genitourinary tracts is truly external blood loss because RBCs are not available for recycling. In these situations, iron is lost through the feces and urine/discharge, respectively. In contrast, hemorrhage into a body cavity or tissue, such as muscle, does not result in iron loss since RBC breakdown products, including iron, are readily available for recycling.
A less common cause of iron deficiency anemia is lack of iron intake, which may affect very young animals receiving a milk and/or cereal-based diet. Piglets are particularly susceptible, given changes in husbandry and lack of access to iron-containing soil. Nursing piglets in today’s facilities are generally given parenteral iron to prevent iron deficiency anemia. Iron lack can be avoided in kittens and puppies by adding solid food to the diet, beginning at 3-4 weeks. Calves may be deprived of iron sources in the diet purposely, in order to produce a pale veal for market.
Iron deficiency anemia is a continuum and, uncorrected, microcytosis (decreased MCV and microcytes noted on smear evaluation), followed by hypochromia, eventually develop. Hemoglobinization of rubricytes and metarubricytes is impaired and the delay in incorporation of iron results in an additional mitotic division in erythroid cells. This additional mitosis is responsible for microcytosis in developing erythrocytes. The lack of iron causes hypochromia in advanced cases, which is identified as pale cells with increased central pallor; fragility and fragmentation of erythrocytes due to the paucity of intracellular hemoglobin; and decreases in MCH and MCHC.
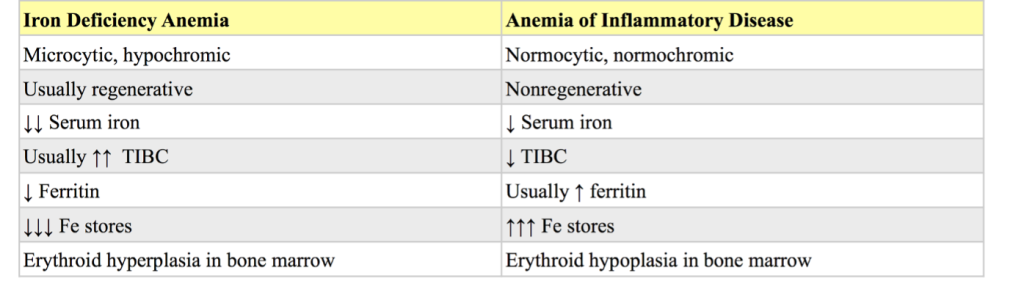
Despite the lack of iron, the anemia is usually regenerative at the time of detection (with the possible exception of anemia due to dietary iron lack in neonatal animals). The regenerative response is generally not as robust as with anemias that are not associated with iron lack, such as hemolysis or internal hemorrhage. Tests which help to confirm iron deficiency are serum iron; total iron binding capacity (TIBC), which is a measure of transferrin, the iron carrier protein; serum ferritin; and visual evaluation of bone marrow iron stores. Serum ferritin and bone marrow examination are the best ways to assess total iron body stores. Serum iron is affected by many conditions besides iron status. Generally, low serum iron and high TIBC concentrations are expected with iron deficiency in most species. However, TIBC is reported to be within RI in affected dogs and cats, but this is an inconsistent finding as cases with high TIBC concentrations are also seen (see additional information, case 4). Although serum iron is also decreased with anemia of inflammatory disease, the TIBC is low to normal with this condition, microcytosis is rare, and hypochromia is not seen. See Table 1.5 for a comparison of findings with iron deficiency compared to the anemia of inflammatory disease.
Serum ferritin assays are not readily available for all species and bone marrow examination is an invasive procedure if iron status is the only concern. Often the history, clinical findings, and peripheral blood findings are sufficient to diagnose iron deficiency anemia. The etiology of the iron lack must then be determined.
Microcytosis Unrelated to Iron Lack
Copper deficiency is a potential cause of microcytic, hypochromic anemia due to its role in iron metabolism. Copper-containing proteins are required for iron transport and their lack may result in functional iron deficiency.
A low MCV is seen in about 2/3 of dogs with portosystemic shunts. Defective iron transport related to decreased hepatic transferrin synthesis is one possible explanation.
Healthy dogs of certain Asian breeds may have lower MCVs than other breeds: Akita, Chow-Chow, Jindo, Shiba Inu, and Shar Pei. Also, normal young calves have low MCVs relative to mature cattle; this finding is not related to iron deficiency.
Initial events in hemostasis, comprising vasospasm/vasoconstriction and platelet plug formation.
General term for a decrease in number of any cell line in peripheral blood.
Disorder of primary hemostasis due to qualitative or quantitative defects in von Willebrand factor. The most common inherited bleeding disorder in dogs.
Anemia in which there is insufficient body iron for effective erythropoiesis, usually caused by chronic external blood loss. Anemia remains regenerative until the end-stage of iron deficiency. The anemia is characterized by microcytic hypochromic RBCs.
Increased number of erythrocytes with decreased volume that usually corresponds with decreased MCV; often associated with iron deficiency as well as portosystemic shunts.
Pale region in the center of an erythrocyte
Equivalent to transferrin concentration; the maximum amount of iron that can be carried in the blood
Storage form of iron within plasma and tissues (soluble).

