Hemolytic Anemia: Immune-Mediated and Oxidative Damage
Hemolytic Anemia
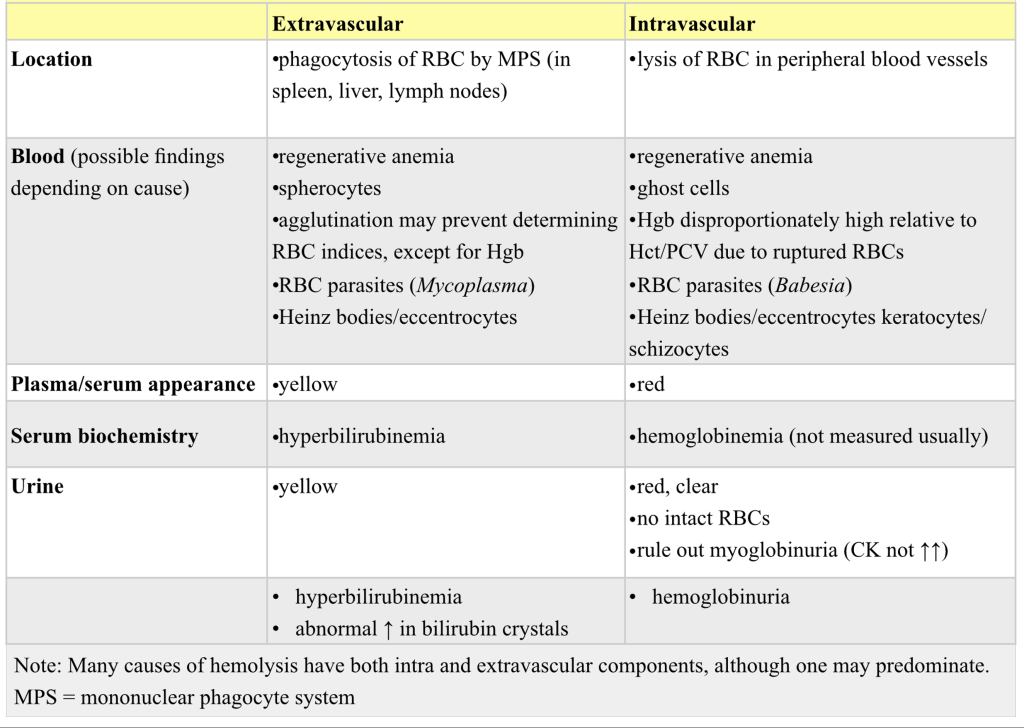
Hemolysis can occur within the peripheral blood (intravascular), in tissues rich in macrophages such as the spleen, liver, and lymph nodes (extravascular, also known as intracellular), and sometimes both. If significant numbers of erythrocytes are lysed in the peripheral blood (intravascular hemolysis), hemoglobinemia and hemoglobinuria are seen. Hemolysis that occurs through the MPS (extravascular hemolysis) may be accompanied by icterus from overwhelming the ability of the liver to take up, conjugate, and/or secrete recycled bilirubin.
Immune-mediated Hemolytic Anemia
A classic and important example of hemolytic anemia that occurs primarily in dogs and is usually idiopathic is immune-mediated hemolytic anemia (IMHA). Affected animals generally become acutely ill and are presented with varying degrees of anemia and RBC regeneration. Extravascular hemolysis occurs when RBCs with surface bound antibody are phagocytosed by macrophages, particularly in the spleen. If the entire RBC is not phagocytosed, a portion of the membrane with bound antibody may be phagocytosed resulting in the formation of spherocytes (Fig 1.9). These small, round, dense erythrocytes are poorly deformable and may be completely phagocytosed on their next exposure to cells of the MPS. The aggressive extravascular destruction of RBCs often results in clinical icterus, hyperbilirubinemia, and hyperbilirubinuria. Intravascular hemolysis may also occur due to complement fixation by antibody and assembly of the membrane attack complex on the RBC surface. Direct damage to the RBC membrane results in leaching of hemoglobin into the plasma, leaving behind a ghost erythrocyte (Fig. 1.10). If the intravascular hemolysis is substantial, then the plasma and urine may be visibly red from hemoglobinemia and hemoglobinuria, respectively. Blood smear findings may include the presence of spherocytes, ghost cells, increased polychromatophilic cells indicating a regenerative response, and possibly agglutination of RBCs due to cross-linking of antibodies coating the erythrocytes (Fig. 1.11). If classic changes are present, additional testing may not be required. However, a direct antiglobulin test (DAT) (also known as a Coombs’ test) or flow cytometry may be performed to detect the presence of antibody, complement, or both, on the surface of the RBCs. See Table 1.1 for examples of RBC morphology that may be seen with IMHA. Also see Figs 1.9-1.11 and Case 11.
Extravascular Hemolysis
In addition to idiopathic IMHA, self or foreign antigens on the surface of RBCs can trigger extravascular hemolysis by the MPS. Sources of foreign antigens that could bind to RBC membranes and elicit an antibody response include drugs, vaccines, viruses, bacteria, parasites, and malignant tumors that produce circulating neoantigens. As an example, certain hemotropic organisms, such as Mycoplasma in cats (Fig. 1.12), dogs, and several other species, and Anaplasma in cattle, may adhere to the RBC membrane triggering the MPS to aggressively remove/phagocytose the infected cells. This results in the development of anemia, often acute, severe, and accompanied by hyperbilirubinemia/icterus.
Intravascular Hemolysis
Intravascular hemolysis is less common than extravascular hemolysis in veterinary medicine. Causes, in addition to the intravascular hemolysis that may accompany IMHA, are bacterial infections (e.g. due to Clostridial organisms), certain erythrocyte parasites (e.g. Babesia spp.), neonatal isoerythrolysis (particularly in horses), excessive water consumption or hypotonic fluid administration, transfusion reactions, RBC metabolic defects (e.g. phosphofructokinase defect in dogs), mechanical shearing of erythrocytes, and hypophosphatemia. Hypophosphatemic hemoglobinuria, also known as postparturient hemoglobinuria, is not restricted to postparturient cattle, nor is it restricted to high producing dairy cattle. Beef cattle on low phosphorus diets (low grain intake), particularly during harsh winter months, can also develop hypophosphatemic hemolysis. Serum phosphorus does not correlate well with whether or not hemolysis occurs. Also, once hemolysis does develop, the serum phosphorus will recover somewhat due to release from ruptured RBCs. To determine if there is a herd problem, clinically normal cattle should be tested since hypophosphatemia may actually be more severe in this group than in the affected animal(s). Phosphorus is a component of ATP which is required for energy within RBCs. With hypophosphatemia, ion pumps become unable to function and RBCs swell and rupture, releasing their contents (hemoglobin) into the circulation. Many of the conditions that result in intravascular hemolysis may also have a component of extravascular hemolysis. See Table 1.6 for features of extravascular and intravascular hemolysis.
Other potential causes of intra- and extravascular hemolysis include congenital red cell defects (e.g. pyruvate kinase defect in dogs and cats), neoplasia of monocyte-derived cells (e.g. histiocytic sarcoma), and oxidative damage to erythrocytes, as described below.
Oxidative Damage
Heinz body hemolytic anemia occurs in all domestic animals, but is probably most common in the dog. Heinz body anemia is due to RBC oxidative injury and may result in intravascular hemolysis, extravascular hemolysis, or both. Oxidative damage to hemoglobin results in precipitation of the denatured globin portion of hemoglobin and the formation of Heinz bodies (see Table 1.1 and Figs 1.13 and 1.14). RBCs containing Heinz bodies are poorly deformable and may be removed when exposed to the MPS, particularly in the spleen (representing extravascular hemolysis). Note: Heinz bodies are common in feline blood and relate, in part, to the nonsinusoidal feline spleen which does not efficiently remove these structures from erythrocytes. Also, feline hemoglobin is more susceptible to Heinz body formation due its content of sulfhydryl groups. When an exogenous oxidizing agent is present and causing hemolysis, the Heinz bodies are large and the anemia becomes regenerative within a few days of the onset of hemolysis.
Lipid peroxidation of the RBC membrane can also occur, resulting in the formation of eccentrocytes (see Table 1.1 and Fig. 1.15). Lipid peroxidation of the RBC membrane may result in sufficient damage that all of the hemoglobin is released from the cells, leaving behind ghost cells (see Table 1.1 and Fig. 1.13). RBC membrane damage and the accompanying release of hemoglobin into the plasma, if of sufficient magnitude, will result in red discoloration of the plasma/serum and urine (hemoglobinemia and hemoglobinuria, respectively).
Methemoglobin production also occurs at a higher rate in the presence of oxidizing agents and the methemoglobin reduction pathway may be overwhelmed. Methemoglobin is unable to bind oxygen. Affected patients have both hemolysis plus the inability of a proportion of their RBCs to deliver oxygen to tissues.
Oxidizing agents include, but are not limited to: onions; zinc; acetaminophen (especially cats); wilted or partially dried red maple leaves (horses); excessive copper; benzocaine (in many topical medications); naphthalene (in moth balls); garlic; skunk spray (on exposed mucous membranes); Brassica-family plants (cattle); and propylene glycol. Certain species may be more likely to ingest or contact these agents than others. Also, there may be both species and individual factors that determine whether or not illness occurs with exposure.
Erythrocyte destruction within blood vessels; often accompanied by hemoglobinemia and hemoglobinuria
Presence of free hemoglobin in the blood, e.g. due to intravascular hemolysis.
Presence of free hemoglobin in the urine, e.g. due to intravascular hemolysis.
Erythrocyte destruction within macrophages of the spleen, liver, lymph nodes, and bone marrow; often accompanied by hyperbilirubinemia and bilirubinuria.
Jaundice; deposition of pigment in skin, mucous membranes and sclera due to hyperbilirubinemia; usually results in a yellow discoloration of skin and mucous membranes.
Break-down product of hemoglobin.
Of unknown cause.
Anemia due to destruction of RBCs that is caused by antibodies produced by the animal’s own immune system. Usually highly regenerative, with presence of spherocytes and agglu
Erythrocyte with a spherical rather than biconcave disk shape, which results in a compact cell with a lack of central pallor. Often associated with immune-mediated hemolytic anemia.
Pale-staining erythrocyte containing very little hemoglobin due to intravascular lysis
Clumping of erythrocytes due to antibody interactions. Must be differentiated from rouleaux formation.
Technique by which cells in fluids (e.g. blood) can be identified based on cell surface markers.
Red cell organism causing hemolytic anemia
Mononuclear phagocytic leukocyte that develops into macrophages in tissue.
Malignant neoplasm of mesenchymal origin, e.g. osteosarcoma.
Erythrocyte in which the hemoglobin is displaced to one side due to oxidative damage to the lipid membrane.

