Secondary Hemostasis (Coagulation)
Secondary hemostasis (coagulation) occurs on the surface of endothelial cells and platelets. Endothelial cells are not constitutively procoagulant but with perturbation, they increase expression or synthesis of several proteins, particularly tissue factor, factor VIIa (“a” indicating the activated form of the coagulation factor), and factor V. Important coagulation protein activation and inhibition reactions also occur on granulocyte and monocyte membranes. For many years coagulation was considered to result from the sequential activation of intrinsic and extrinsic factors in a waterfall or cascade model converging into a final common pathway. This model remains important in understanding coagulation test results, but does not truly reflect the in vivo event.
Coagulation factors were assigned Roman numerals in the order in which they were discovered (Table 4.1). Some of these are no longer referred to by their numeral, but by their common name. For example, factor I is usually called fibrinogen. Many factors were originally named according to the factor-deficient family of their first description. For example, Fletcher factor, named after the sentinel family with factor-deficient members, is now generally referred to as prekallikrein. Although factor IV is calcium, the Roman numeral is almost never used.
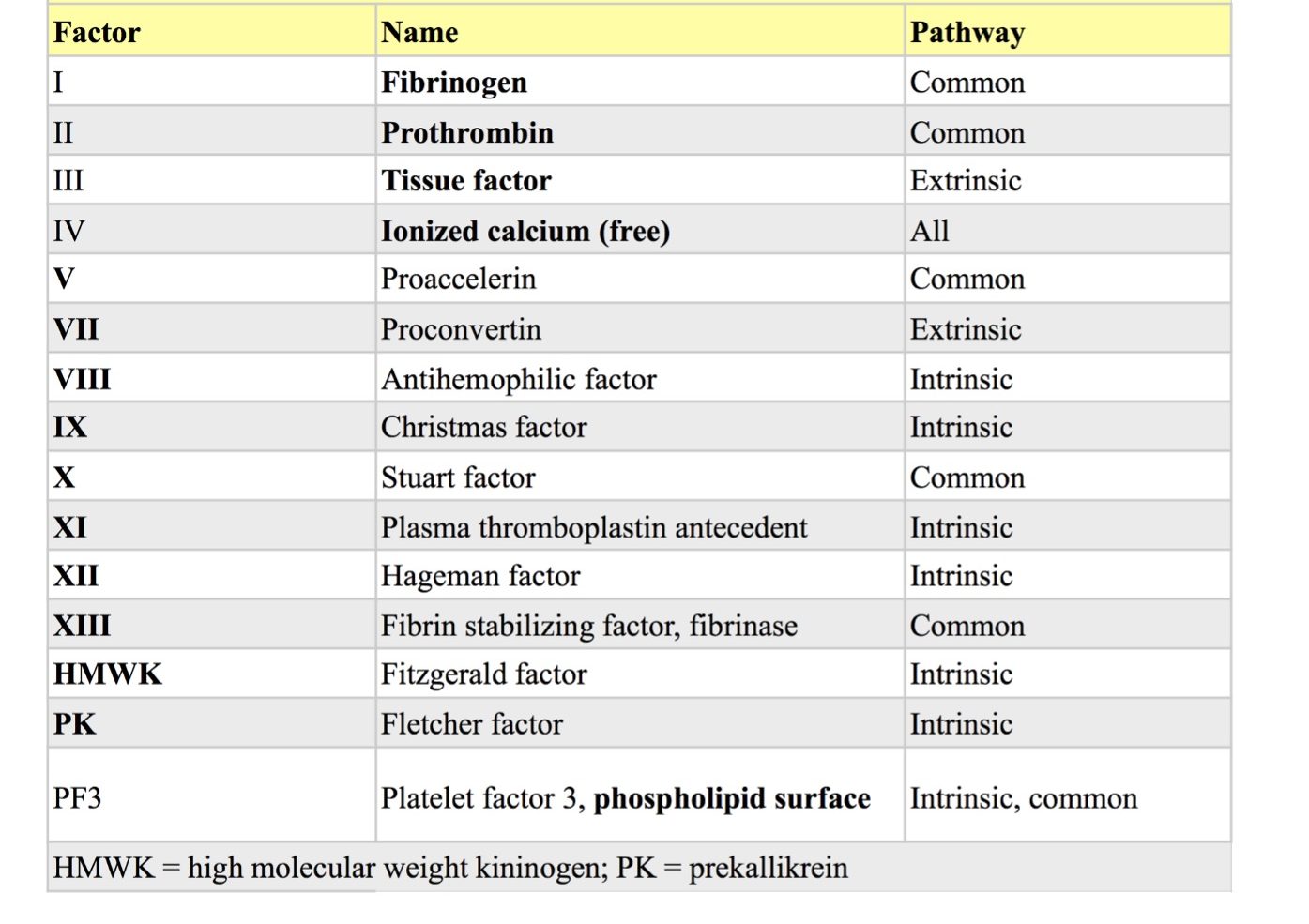
Several years ago the traditional cascade theory of coagulation was revised to a 3-stage, cell-based model of coagulation. This model emphasizes the interrelatedness of the intrinsic and extrinsic systems in vivo, and the importance of cell surfaces (particularly those of platelets and endothelial cells) in anchoring and limiting the process. Although deficiencies of the contact factors are not generally associated with bleeding tendencies, bleeding has been reported, though rarely, in canine and equine prekallikrein-deficient patients, similar to factor-deficient members of the Fletcher family. The contributions of intrinsic factors VIII and IX to normal coagulation are extremely important as patients with deficiencies of these (hemophilia A and B, respectively), experience life-threatening bleeding.
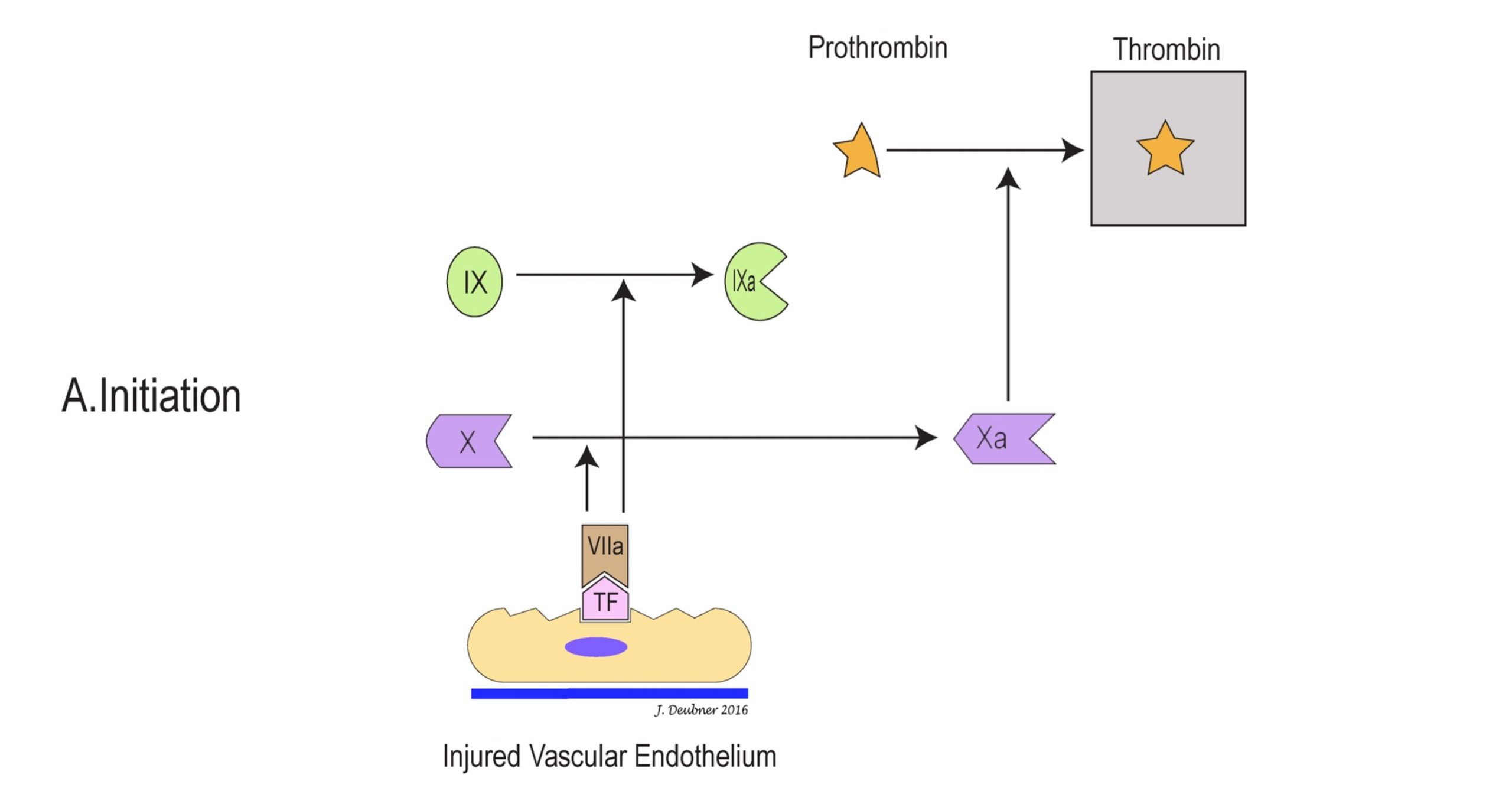
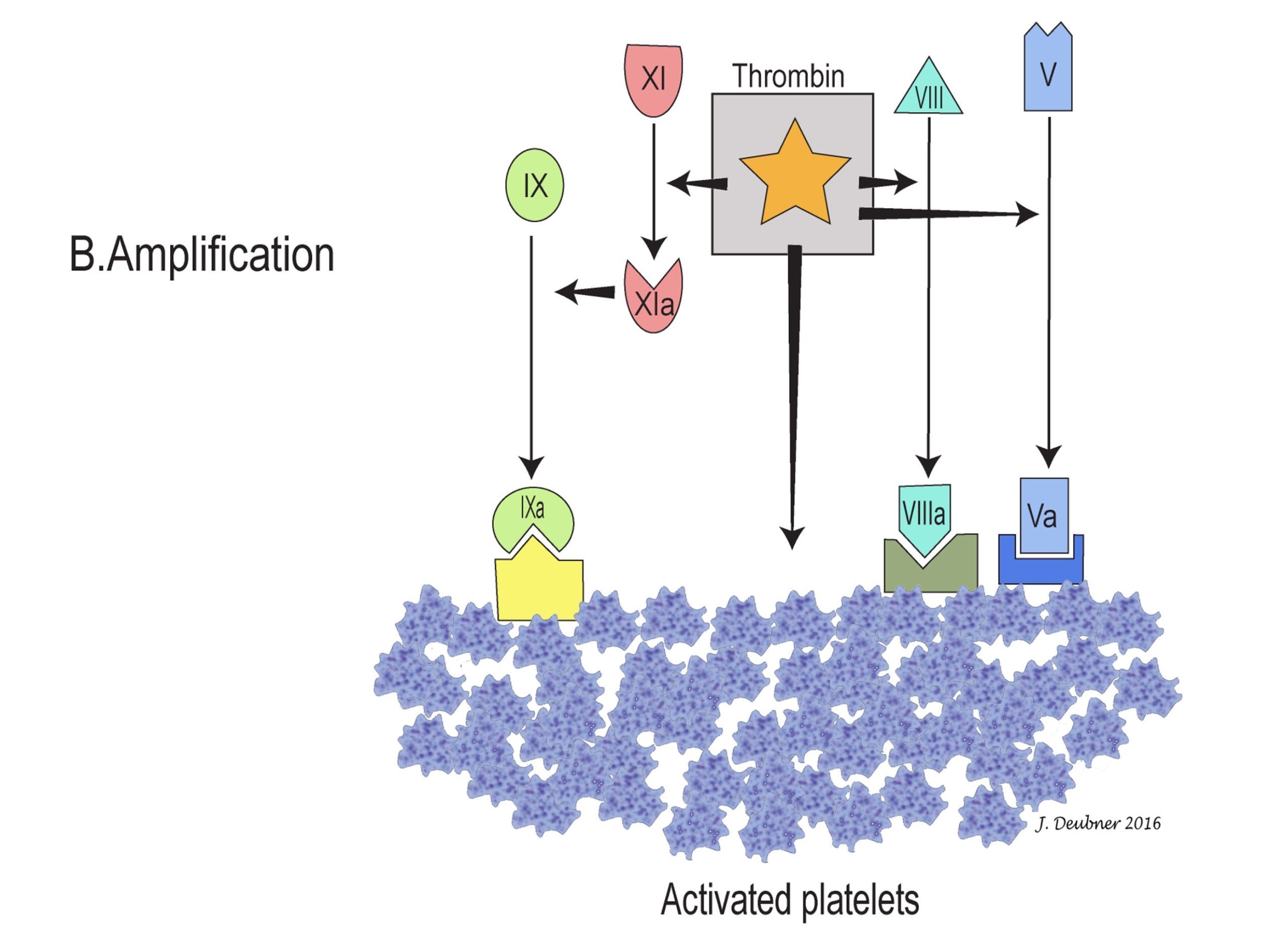
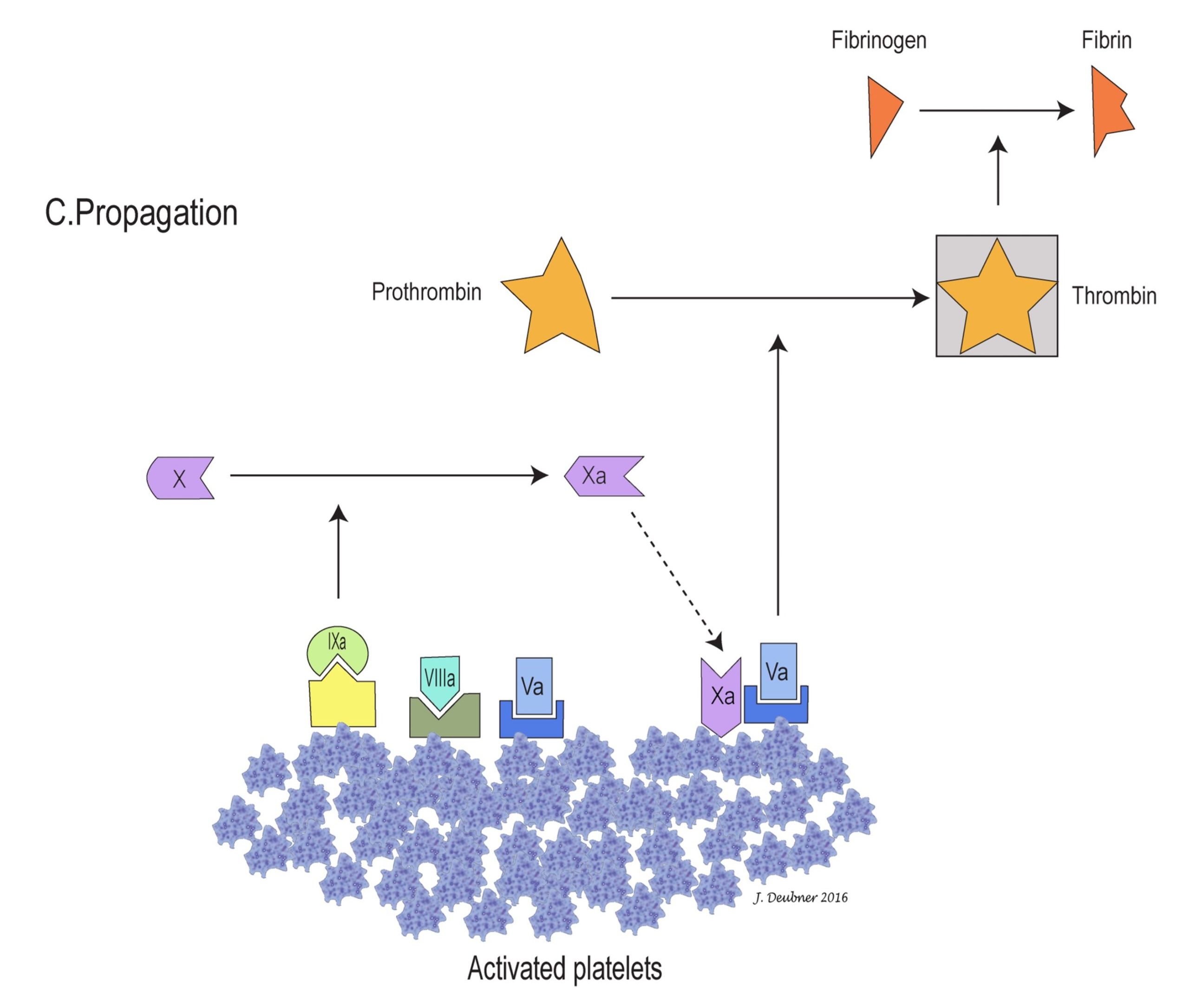
In the cell-based model, coagulation can be divided into three stages: initiation, amplification, and propagation (Figs. 4.4A to 4.4C). The initiation phase involves primarily the extrinsic system and generates only small amounts of thrombin. In the amplification phase, the available thrombin activates factors XI, VIII, V, and platelets. Factor XIa activates additional factor IX, which, together with factors VIIIa and Va on platelet surfaces, sets the stage for the propagation phase by generating large amounts of factor Xa. Factor Xa ultimately converts prothrombin to thrombin and fibrinogen to fibrin, the goal of coagulation. The phospholipid wall of platelets and other cells provides the surface for activation and assembly of coagulation factors and cofactors. Propagation of coagulation occurs because the stage is thus set for major fibrin formation.
The following disorder underscores the importance of the interaction between primary hemostasis and secondary hemostasis demonstrated by the three-stage, cell-based model of coagulation. A hereditary bleeding disorder caused by lack of platelet procoagulant activity has been identified in German Shepherd dogs. Affected dogs may experience hemorrhage associated with surgery as well as less invasive activities (e.g. epistaxis, soft tissue hemorrhage, hyphema, and hemorrhage associated with dental procedures). All standard tests of primary and secondary hemostasis are within RI. The disorder, called canine Scott Syndrome, after its counterpart in human medicine, is due to an inability of affected platelets to support assembly of the coagulation proteins that ultimately generate thrombin. The diagnosis is made using non-routine/specialized tests that evaluate platelet procoagulant activity.
In vitro versus in vivo coagulation
Although the 3-stage, cell-based model of coagulation best reflects coagulation in vivo, an understanding of the cascade model is helpful when considering the clinical evaluation of coagulation. The term intrinsic reflects the fact that blood will clot on its own when added to a tube; all the factors required for coagulation to occur are contained in (intrinsic to) the blood. This in vitro process can take several minutes, particularly if the inner surface of the tube is siliconized and, therefore, positively charged. On the other hand, coagulation occurs at a rapid rate in vivo when blood is exposed to damaged tissue suggesting that a factor not normally present in (extrinsic to) the blood is required for the process. The intrinsic pathway is activated by exposure of contact factors (HMWK, prekallikrein, and factor XII) to a surface. In vivo, the surface is negatively charged subendothelium, which is only exposed with endothelial injury. The extrinsic pathway is activated by synthesis and release of tissue factor from damaged tissues. Obviously, these two pathways do not operate independently. However, the activated partial thromboplastin time (APTT, henceforth PTT) and one stage prothrombin time (OSPT, henceforth PT) form the basis of diagnostic testing of coagulation. The PTT evaluates the intrinsic and common pathways, and the PT evaluates the extrinsic and common pathways (Fig. 4.5).
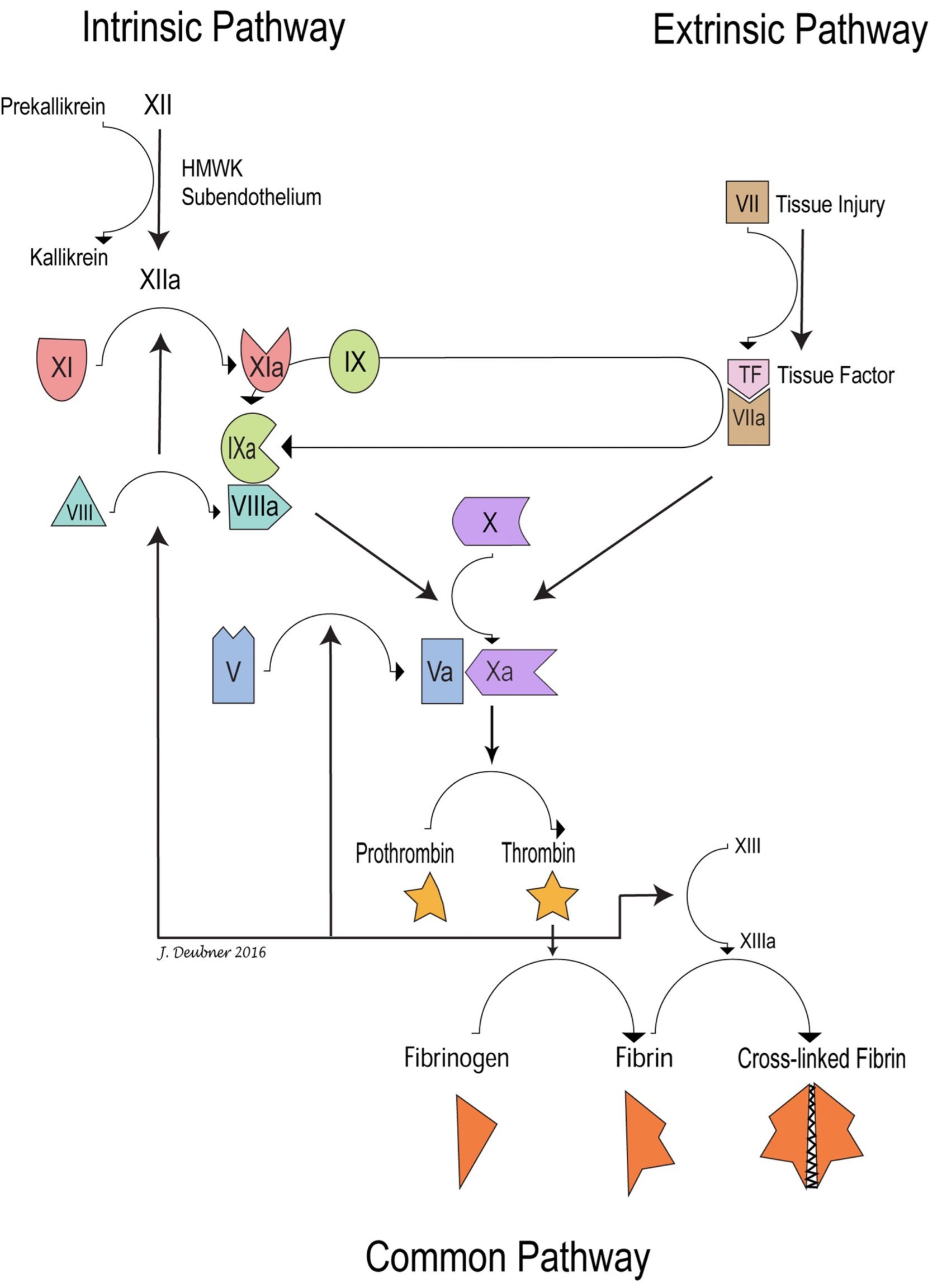
The PTT is measured by adding calcium, a contact activating agent (such as kaolin or diatomaceous earth), and platelet phospholipid, to citrated plasma. Calcium and tissue factor are added to citrated plasma to measure the PT. Tissue factor is an integral membrane protein which is expressed on many extravascular tissues, but is not normally expressed on vascular endothelium. In vivo, various inflammatory cytokines and bacterial by-products can induce tissue factor expression on monocytes and vascular endothelial cells. The PTT generally takes more time than the PT because there are many more steps between contact factor activation and fibrin formation compared to factor VII activation and fibrin formation in the extrinsic system.
The PTT and PT do not evaluate the interactions between intrinsic and extrinsic systems and the PT can proceed rapidly without factors IX and VIII, which are so important in vivo. In vitro testing of the extrinsic system by PT does not require these factors because a large amount of tissue factor is added when performing the test. In vivo, often a localized small amount of tissue factor is available initially, and this complexes with and activates factor VII. Factor VIIa not only activates factor X, but also activates factor IX. In the conversion of factor X to Xa, factor IXa provides enzymatic activity, factor VIIIa is a reaction accelerator, and factor X is the substrate. Without the products of the intrinsic system, coagulation proceeds very slowly in vivo, which explains why individuals with factor IX or factor VIII deficiency have severe bleeding tendencies. On the other hand, patients with very low concentrations of factor VII do not experience significant bleeding. A possible explanation for this finding is that even with very low concentrations of this protein, there is still sufficient to complex with and activate factor X.
An important link between intrinsic and extrinsic systems is that, in addition to activating factor X, the factor VIIa/tissue factor complex also activates factor IX. The extrinsic pathway is probably short-lived in vivo due to specific inhibition of factor VIIa-tissue factor activation of factor X. However, this inhibition does not affect factor VIIa-tissue factor activation of factor IX. Thus, activation of factor X continues due to intrinsic pathway contributions by factors IXa and VIIIa.
Mobile leukocyte with the ability to phagocytose, degrade, and/or kill microorganisms (neutrophil, eosinophil, basophil).
Mononuclear phagocytic leukocyte that develops into macrophages in tissue.
Traditional model of coagulation comprising sequential activation of clotting factors in the intrinsic, extrinsic, and common pathways
Final component of the cascade model of coagulation; initiated by the activation of factor X by both the intrinsic and extrinsic pathways and terminating in the production of cross-linked fibrin.
Factor involved in initiating the intrinsic pathway: HMWK, prekallikrein, and factor XII.
Defective secondary hemostasis due to a lack of clotting factors VIII or IX
Central protein of coagulation; not only converts fibrinogen to fibrin, but also plays important roles in amplification and fibrinolysis.
Component of the cascade model of coagulation initiated by exposure of contact factors to subendothelium and terminating in the common pathway. PTT evaluates the intrinsic and common pathways.
Component of the cascade model of coagulation initiated by release of tissue factor from damaged tissue and terminating in the common pathway. Prothrombin time (PT) evaluates the extrinsic and common pathways.
Activated Partial Thromboplastin Time; see PTT.
Prothrombin time; diagnostic test used to evaluate the extrinsic and common pathways.
Factors V and VIII decrease the time required for coagulation to occur.

