Routine Tests of Muscle Damage
Routine biochemical panels and urinalyses generally include several tests that assess muscle damage according to leakage of enzymes and proteins from injured muscle tissue. These markers of muscle leakage are not abnormal with many muscle disorders, particularly those involving primary functional defects, atrophy, or neoplasia. Muscle disorders causing inflammation and necrosis are most likely to result in elevations in enzyme activity. There are various types of muscular dystrophies, some of which are associated with increased enzyme activities and others which are not.
Creatine kinase (CK)
CK is present in all types of muscle, brain, and nerves. However, increased serum CK activity is of muscle cytosol origin, primarily skeletal and cardiac. CK catalyzes the conversion of creatine phosphate to creatine and ATP in order to provide an energy source for active muscles. During periods of inactivity, CK catalyzes the reverse reaction to provide an energy reservoir. Creatinine is the metabolic waste product of creatine degradation.
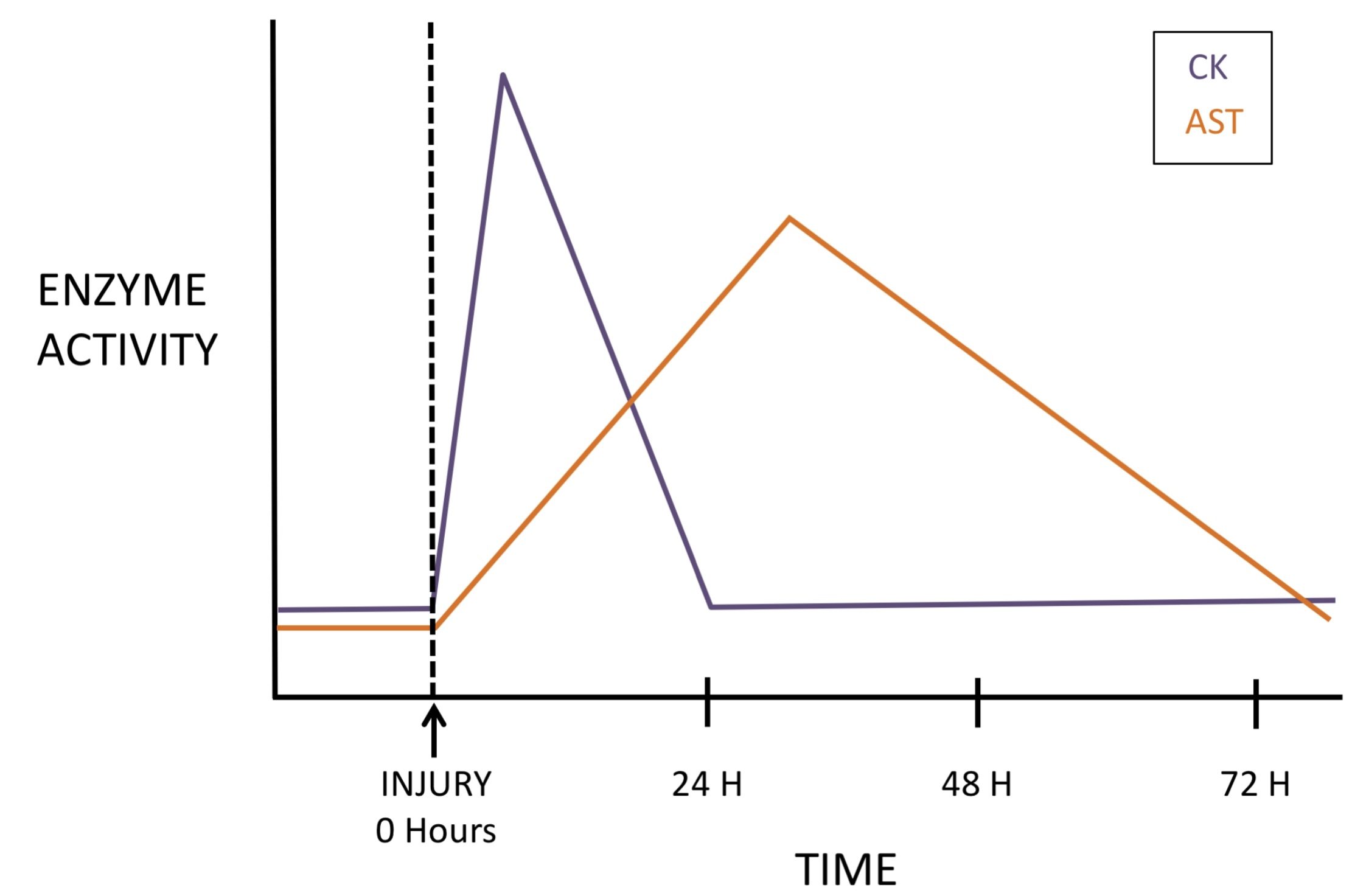
CK serves as an enzyme marker for myofiber damage and is used as an aid for muscle disease diagnosis. CK activity increases rapidly (peaking within hours) and declines rapidly (returning to baseline within 2-3 days) following a single muscle insult. The half-life of CK is reported to be approximately 2 hours in dogs and horses. The magnitude of increased CK activity does not indicate whether the injury is reversible or irreversible, the extent of muscle mass involved, or the etiology of the injury. Mild to moderate increases in CK activity that persist indicate ongoing injury/disease, and may be much more serious than an extremely high level that quickly declines.
Some of the most common conditions associated with increased CK activity in veterinary medicine are: trauma (such as vehicular accidents and bite wounds), prolonged recumbency (especially in large animals), vitamin E/selenium deficiency (white muscle disease), exertional rhabdomyolysis (including capture myopathy), repeated seizure activity, transport (especially in large animals), arterial thromboembolism (with muscle necrosis), and intramuscular injections. Increased CK activity is also frequently associated with venipuncture, either due to intense physical activity and restraint prior to and during the procedure, or due to difficulties localizing the vein and inadvertently puncturing muscle tissue in the area.
Exertional rhabdomyolysis in horses is often referred to as “tying up” and affected animals have muscle stiffness and pain after exercise. Tying up is a general term with several different causes and may be sporadic or recurrent. Recurrent episodes of tying up are thought to have a genetic link, as with recurrent exertional rhabdomyolysis in Thoroughbreds and polysaccharide storage myopathy (PSSM) in Quarter Horses and draft breeds. PSSM is a glycogen storage disease associated with accumulation of increased glycogen and abnormal polysaccharide inclusions in muscle fibers. A genetic mutation is responsible for the condition in horses with PSSM type 1, while horses without the mutation are classified as PSSM type 2.
CK is a dimer, and 3 main isoenzymes exist based on different dimer pairs. Perhaps because myocardial infarction, as a primary event, is rare in domestic animals, CK isoenzyme determination has not been shown to be useful or practical in veterinary medicine.
Aspartate aminotransferase (AST)
Although AST is present in almost all cells, increases in serum activity are of liver or muscle origin, or both. With muscle injury, AST increases more slowly than CK (peaking at 24-36 hours) and declines more slowly (over several days, with wide species variations; Fig. 11.1). In contrast to CK, the half-life of AST is notably longer, approximately 1 day in dogs and 7-8 days in horses. Therefore, a few days following a single muscle insult, CK activity may be within the RI, while AST activity remains elevated. Evaluating AST activity relative to sorbitol dehydrogenase (SDH) activity helps determine if the AST is of liver or muscle origin, since SDH is liver specific.
Alanine aminotransferase (ALT)
ALT is considered to be primarily a hepatic enzyme; however, severe muscle injury is occasionally associated with increases in ALT activity in small animals. The half-life of ALT is longer than AST. Both CK and ALT activities are often increased following vehicular trauma in dogs, and concurrent determination of SDH activity may be necessary to determine if only muscle injury or both muscle and hepatic injury have occurred.
Potassium (K+)
K+ is primarily an intracellular electrolyte and one would suspect that massive muscle damage resulting in increases in leakage enzyme activities would also be associated with release of K+ into the serum. However, this rarely occurs and there is little correlation between serum muscle enzyme activities and potassium concentrations. Hereditary hyperkalemic periodic paralysis (HYPP), usually precipitated by exercise, has been described in Quarter Horses and dogs. In Quarter Horses, a mutation in the skeletal muscle sodium channel gene has been identified. Affected horses have paralytic attacks that are caused by increased serum K+; however, serum K+ is within the RI between episodes and CK activity is within the RI or only mildly elevated. There is only a single report of HYPP in a dog, an American pit bull that experienced weakness after exercise in association with prolonged elevations of serum K+. The final diagnosis was made when the dog experienced weakness following oral potassium administration and responded well to the typical human HYPP treatment of acetazolamide (which promotes renal loss of potassium).
Both acquired and hereditary hypokalemic polymyopathy have been described in cats. Certain diets and diseases, such as renal failure, have been associated with the acquired form of the disease. A correction in formulation of feline diets or supplementation with oral potassium reverses the clinical signs of weakness, ventroflexion of the neck, and stilted gait. Affected animals have increased serum CK activities in addition to hypokalemia.
Myoglobin
Myoglobin is a heme protein that is responsible for oxygen transport in muscle. Release of myoglobin occurs when muscle injury is severe and acute, often involving degeneration and necrosis. Myoglobin is not bound to protein in the plasma and is quickly filtered by the kidneys resulting in dark red to brown discoloration of the urine, without plasma discoloration.
Red plasma in combination with red urine suggests hemoglobinemia and hemoglobinuria, whereas, clear plasma and red-brown urine suggest myoglobinuria. Special urine filtration techniques can be used that separate the larger hemoglobin molecule from the small myoglobin molecule. The filtrate can then be applied to a urinalysis test strip knowing that hemoglobin has been eliminated from the sample. Hematuria can be differentiated from myoglobinuria based on the presence of high numbers of erythrocytes on urine sediment examination. Several other methods are available to confirm the presence of myoglobin in urine.
Abnormal uncontrolled growth of cells that are unresponsive to normal physiologic growth controls; may be benign or malignant.
Enzyme that is released into the peripheral blood following muscle injury.
Heme protein responsible for oxygen transport in muscle.
Presence of free hemoglobin in the blood, e.g. due to intravascular hemolysis.
Presence of free hemoglobin in the urine, e.g. due to intravascular hemolysis.
Oxygen-carrying molecule within erythrocytes.
Red blood cell (RBC); an anucleate (in mammalian species) cell containing hemoglobin needed for oxygen transport. Typically shaped like a bi-concave disk.

