Neutrophil Dysfunction and Morphologic Abnormalities
Defects of neutrophil function are uncommon in domestic animals, but can be a cause of recurrent, sometimes life-threatening, infections.
Normal neutrophil function
Neutrophils in the marginating pool of the peripheral circulation roll along the endothelium under normal conditions.When both endothelial cells and neutrophils are stimulated, neutrophils increase the number of receptors for selectins which are up-regulated on the surface of endothelial cells. The initial interaction is discontinuous; however, as chemoattractants are released from inflamed tissues, integrins facilitate more stable adhesion between endothelium and neutrophils. Subsequently, the adhesion is lessened by several chemicals, enzymes, and cytokines acting locally, which allows neutrophils to migrate through the endothelial barrier at intercellular junctions. Neutrophils then respond to chemotactic mediators released from the site of inflammation or infection and migrate to their destination. Neutrophils phagocytose microbes and tissue debris, and kill or degrade the phagocytosed material by release of granule contents into phagolysosomes and production of reactive oxygen species. Defects at any stage of this process can result in ineffective responses to invaders.
Neutrophil dysfunction
Both acquired and hereditary defects of various neutrophil functions have been described. Most are associated with chronic, recurrent infections. Clinicopathologic findings will depend on the particular function that is impaired, and diagnosis of the problem usually involves specialized tests which are beyond the services provided by most diagnostic laboratories. For example, a leukocyte adhesion defect has been described in cattle and dogs, bovine leukocyte adhesion deficiency (BLAD) and canine leukocyte adhesion deficiency (CLAD), respectively. Affected animals develop chronic infections and rising neutrophil counts because of chronically increased demand for neutrophils in the face of an inability of neutrophils to adhere to endothelium and migrate into sites of inflammation.
Chédiak-Higashi syndrome is a hereditary disease caused by mutation of a lysosomal trafficking gene resulting in dysfunction of several cell types, including neutrophils. Signs include variable susceptibility to infections. Morphologic abnormalities result from accumulation and lack of mobilization of lysosomal granule contents. Large cytoplasmic granules containing lysosomal enzymes may be seen in platelets, natural killer lymphocytes, as well as neutrophils on peripheral blood smear examination (Fig. 2.6). This rare condition has been reported in blue smoke Persian cats, certain breeds of cattle, and several non-domestic species.
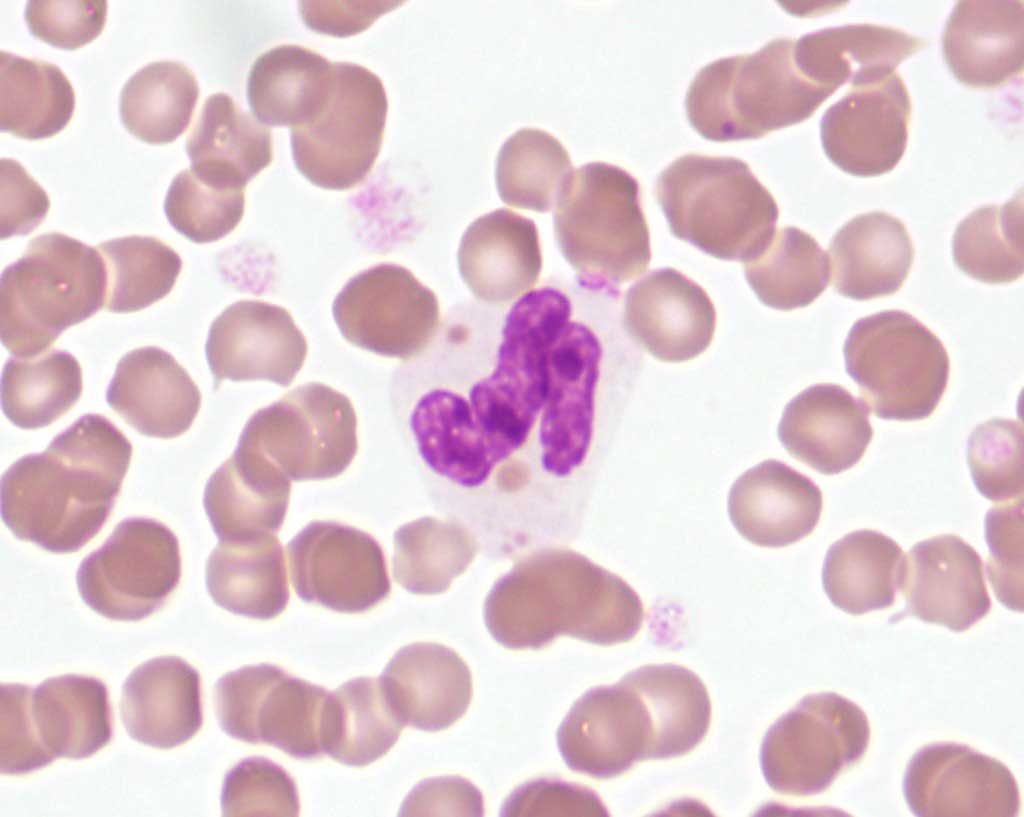
Pelger-Huët anomaly is an inherited disorder resulting in hyposegmentation of granulocyte nuclei such that bilobed, band-shaped, or round nuclei are seen in these cells, which are otherwise mature, functional, and display no toxic change (Fig. 2.7). Nuclear hyposegmentation is also seen in monocytes and megakaryocytes, which is less obvious than the abnormality in granulocytes. The condition is not of clinical significance in heterozygotes, but can be misinterpreted as a severe left shift associated with inflammation. This anomaly has been identified in several species including dogs, cats, and horses.
Polyploid cell found in the bone marrow (also spleen and lung) that produces platelets.

