Metabolic Alkalosis
Metabolic alkalosis occurs when bicarbonate accumulates in the ECF. Metabolic alkalosis is usually produced by loss of H+ from the ECF. HCO3– and pH are both increased. The biochemical panel usually demonstrates increased bicarbonate, hypochloremia (due to loss or sequestration in the stomach or abomasum along with H+), and normal anion gap.
Causes of metabolic alkalosis: sequestration or loss of HCl, usually due to loss of gastric or abomasal HCl leading to a net gain in HCO3– through local HCO3– production. This HCO3–, in turn, is absorbed through the gastric/abomasal wall into the bloodstream (Fig. 6.5).
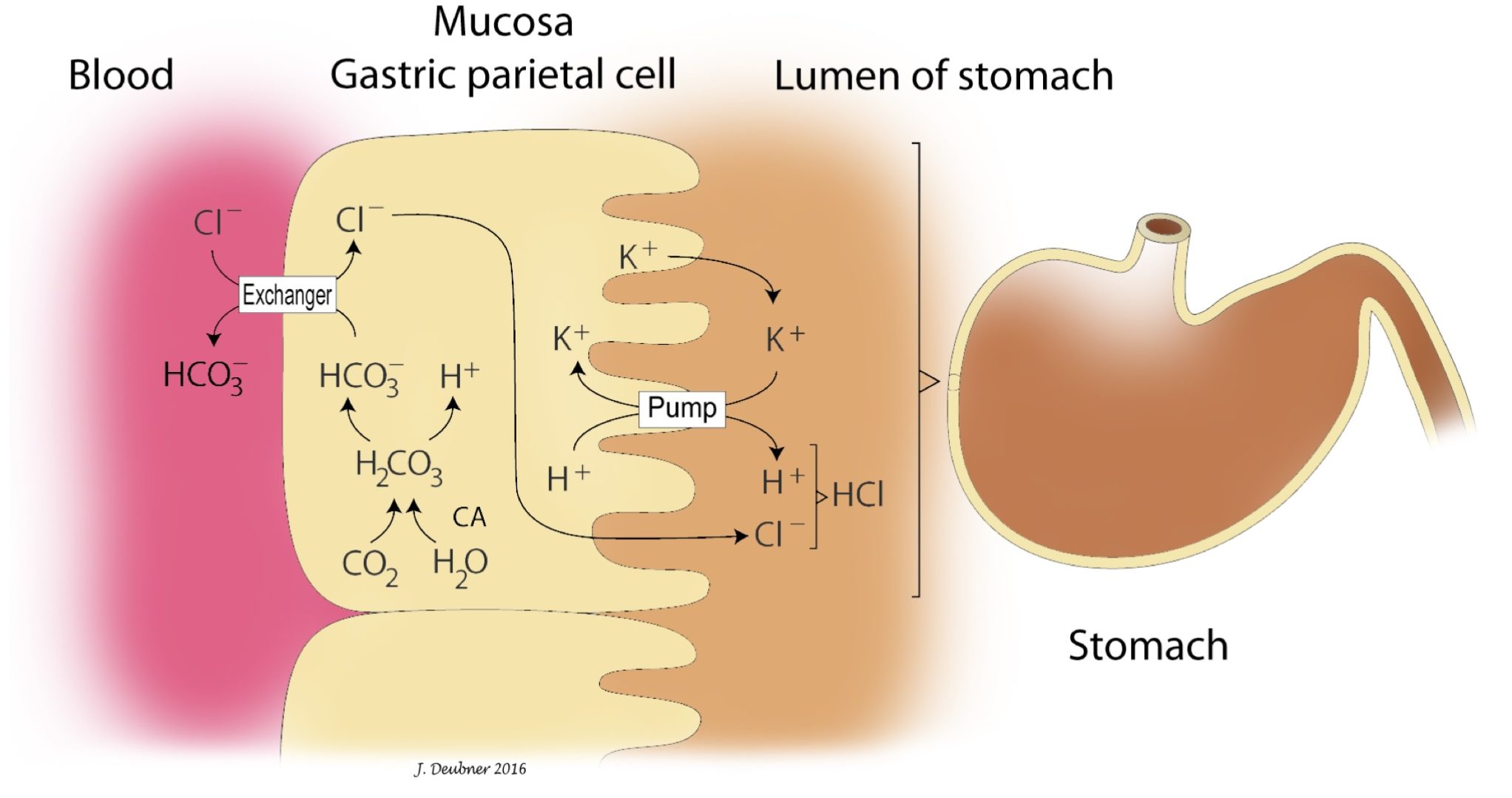
In addition, HCO3– secreted by the pancreas is reabsorbed in the intestines because no H+ is available to bind to it (normally comes from HCl secreted into the stomach), further exacerbating the metabolic alkalosis. Loss or sequestration of HCl occurs with abomasal disorders (e.g. stasis, displacement, and torsion) in ruminants. In monogastric species, metabolic alkalosis may result from prolonged vomiting (particularly if restricted to the stomach and upper small intestine), high intestinal obstructions (due to foreign bodies, tumors, or severe swelling/inflammation), or occasionally with slow moving intestinal foreign bodies regardless of the location.
Metabolic alkalosis can also occur iatrogenically through excessive administration of bicarbonate-rich fluids, magnesium hydroxide (MgOH) administered orally (in ruminants, likely due to alkalinizing effects in the rumen), or diuretics (ECF volume depletion occurs which is countered by increased aldosterone production; K+ loss is promoted by the kidneys, HCO3– is generated and absorbed resulting in metabolic alkalosis).
Respiratory compensation
Respiratory compensation for metabolic alkalosis occurs by hypoventilation. Respiratory compensation is identified by an increase in pCO2. The expected respiratory compensation for metabolic alkalosis is an increase of 0.7 mm Hg pCO2 for every 1.0 mmol/L increase in HCO3–. If the pCO2 is low or normal then a mixed acid-base disorder (i.e. metabolic alkalosis and respiratory alkalosis) is likely.
Paradoxic aciduria
Paradoxic aciduria is a situation in which an animal with metabolic alkalosis (who would be expected to retain H+) instead loses H+ in the urine, resulting in aciduria. Paradoxic aciduria sometimes develops in instances of prolonged metabolic alkalosis with hypochloremia and intravascular fluid volume depletion. When chloride is depleted, bicarbonate has to be reabsorbed instead along with sodium in the proximal tubules in order to maintain electroneutrality. Under these circumstances, Na+ is avidly reabsorbed to try to restore normal intravascular fluid volume; Na+ is reabsorbed in exchange for H+ which passes into the urine (making it acidic). K+ is usually also depleted in this situation, therefore, it is not available to be excreted in exchange for Na+. The reabsorption of HCO3– and excretion of H+ is the opposite of what is normally expected in states of metabolic alkalosis.

