Fibrinolysis and Anticoagulation
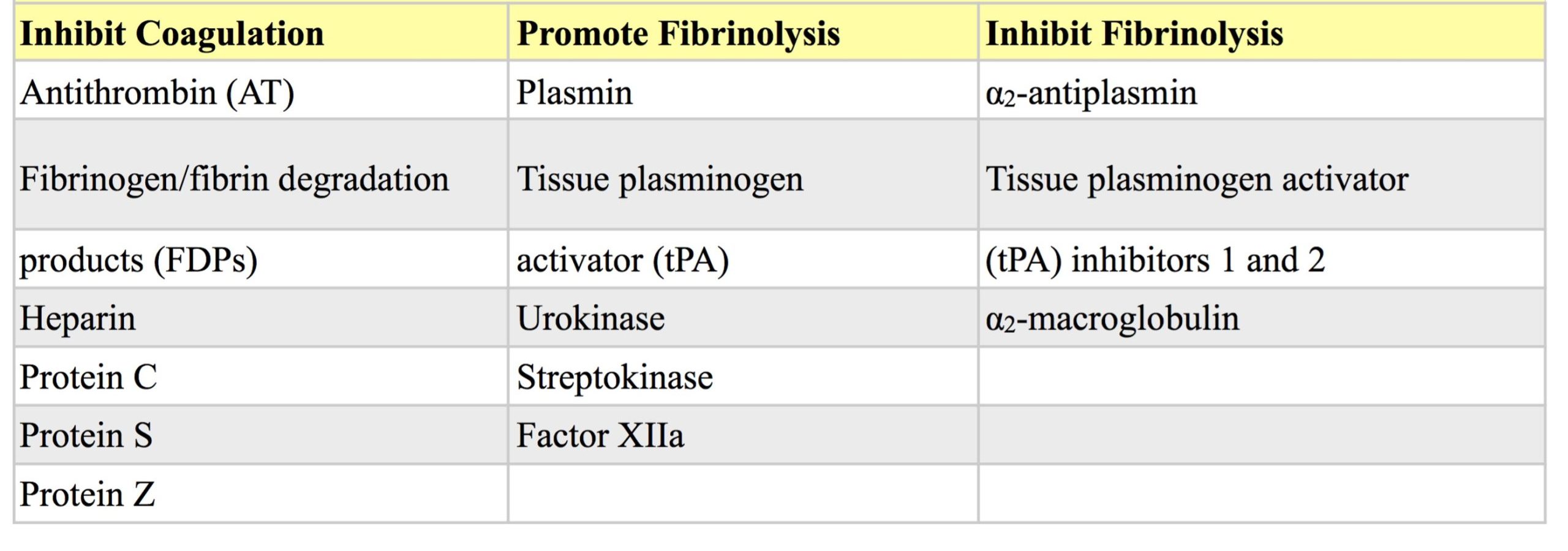
Fibrinolysis is normally a tightly controlled process initiated by incorporation of plasminogen into the fibrin clot (Fig. 4.6). Various plasminogen activators in the blood and endothelium diffuse into the clot and convert plasminogen into the potent proteolytic enzyme, plasmin. These plasminogen activators include: tissue plasminogen activator (tPA), urokinase, streptokinase, and factor XIIa. Antiplasmin in the plasma rapidly neutralizes plasmin as it is formed and therefore, restricts fibrinolytic activity to the localized region of the dissolving fibrin clot. In addition to digesting fibrin polymers, plasmin also degrades fibrinogen, and factors II, V, VIII, and XII. Fibrinolysis is inhibited by α2-antiplasmin, α2-macroglobulin, and tPA inhibitors 1 and 2.
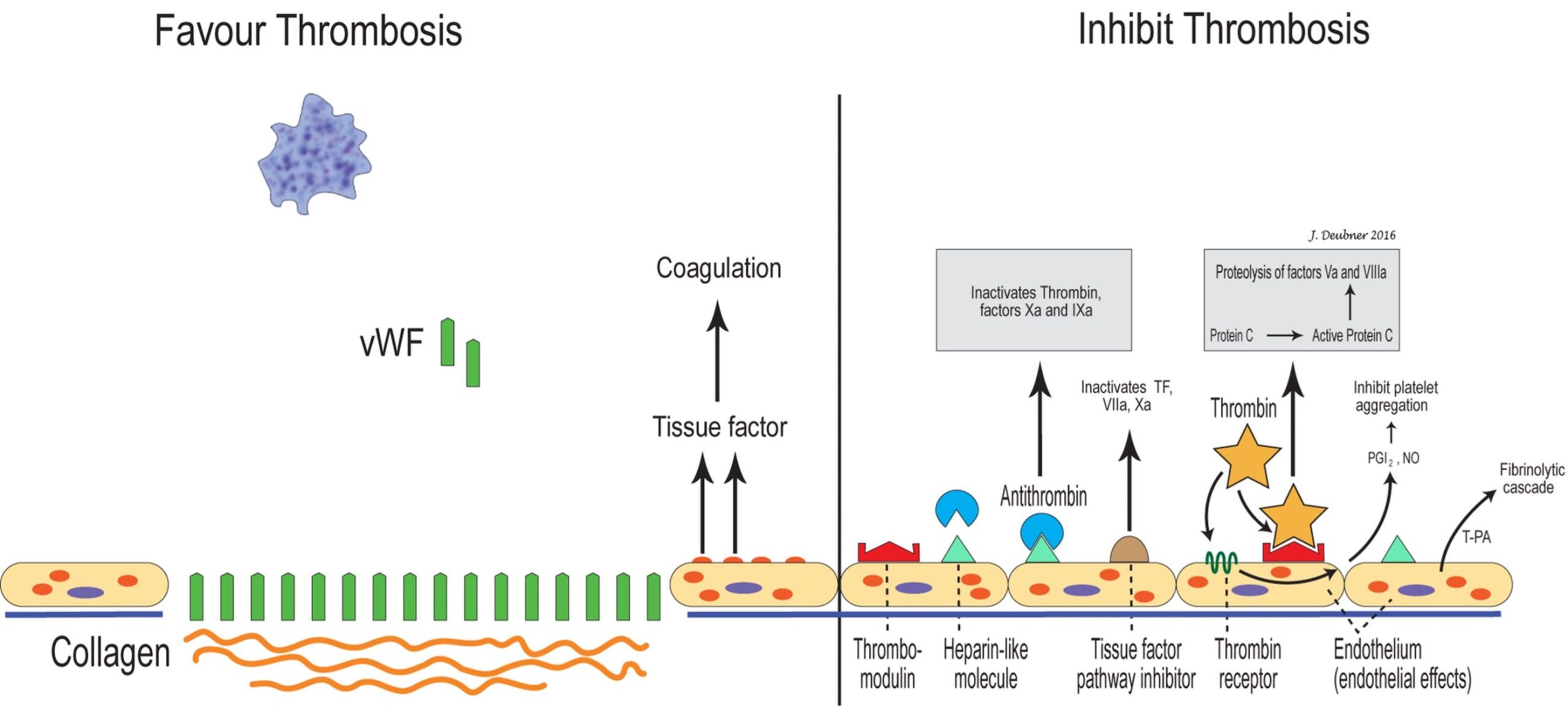
Coagulation can also be limited in other ways. Many activated coagulation factors initially promote coagulation but later inhibit coagulation. Fibrinogen/fibrin degradation products (FDPs) inhibit platelets, thrombin, and fibrin polymerization. Antithrombin (AT) inhibits many activated factors in addition to thrombin; heparin is a cofactor for AT and greatly increases its efficiency. Protein C is a vitamin K dependent enzyme which inactivates factors Va and VIIIa. Protein S is also a vitamin K dependent protein which acts as a cofactor for binding of activated protein C to cell surfaces. Protein Z, also vitamin K dependent, is involved in the degradation of factor Xa through protein Z-related protease inhibitor (PZI) by greatly accelerating the activity of the protease inhibitor. Although first identified in bovine plasma, little is available in the veterinary literature about protein Z. Factors involved in regulating coagulation are presented in Table 4.2 and Fig. 4.7.
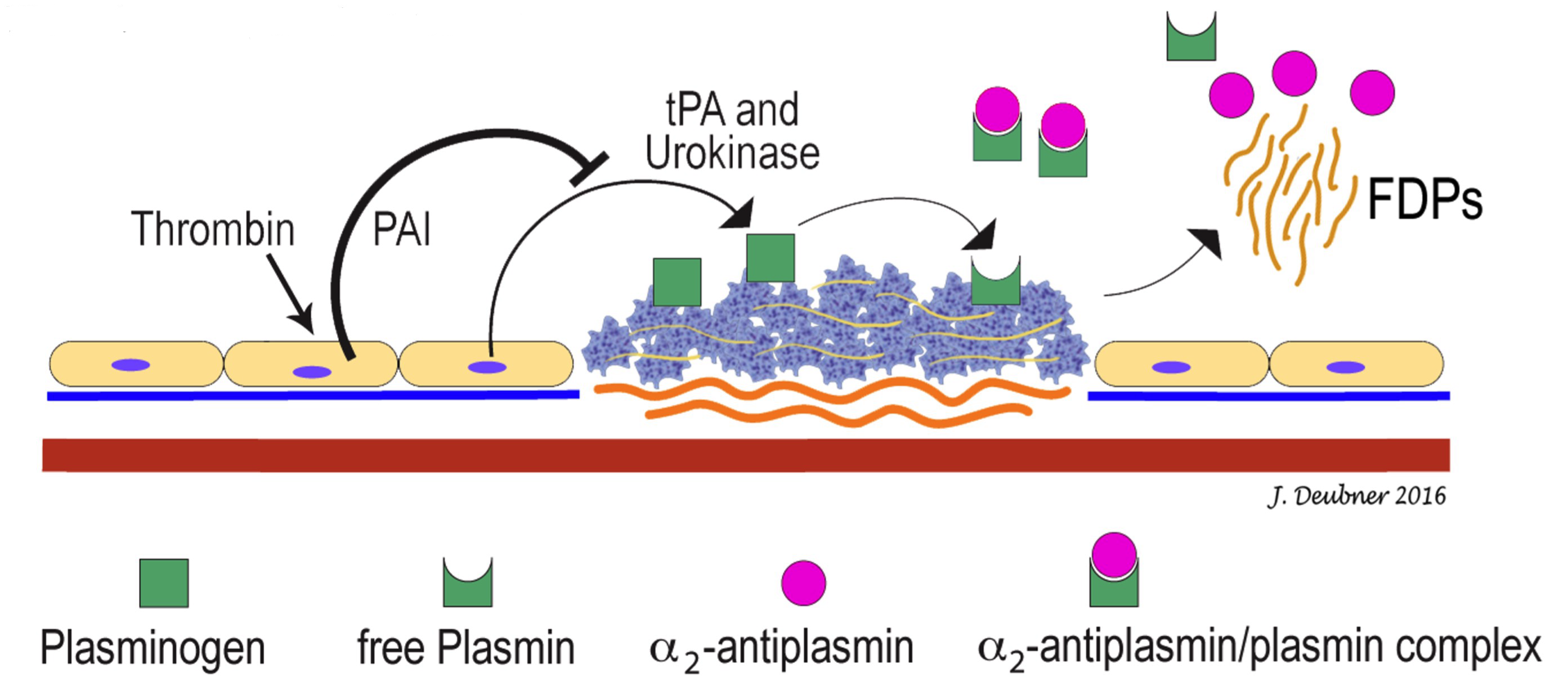
Proteolytic enzyme important in fibrinolysis.
Fibrin/fibrinogen Degradation Products; increased concentration of these products supports a diagnosis of DIC&F.

