Erythrocyte Metabolism
As mature mammalian RBCs are anucleate and lack mitochondria, they are incapable of oxidative phosphorylation and DNA, RNA, and protein synthesis. Glucose is the primary energy source for RBCs in most species. Erythrocyte energy needs are met primarily through anaerobic glycolysis and the generation of adenosine triphosphate (ATP) which allows RBCs to carry out functions including, but not limited to: delivery of oxygen to and removal of carbon dioxide from tissues; buffering hydrogen ions in the circulation; and maintaining physical characteristics of deformability and shape. Additional metabolic pathways within RBCs regulate oxygen release to tissues, and provide protection from oxidative damage.
Energy Production
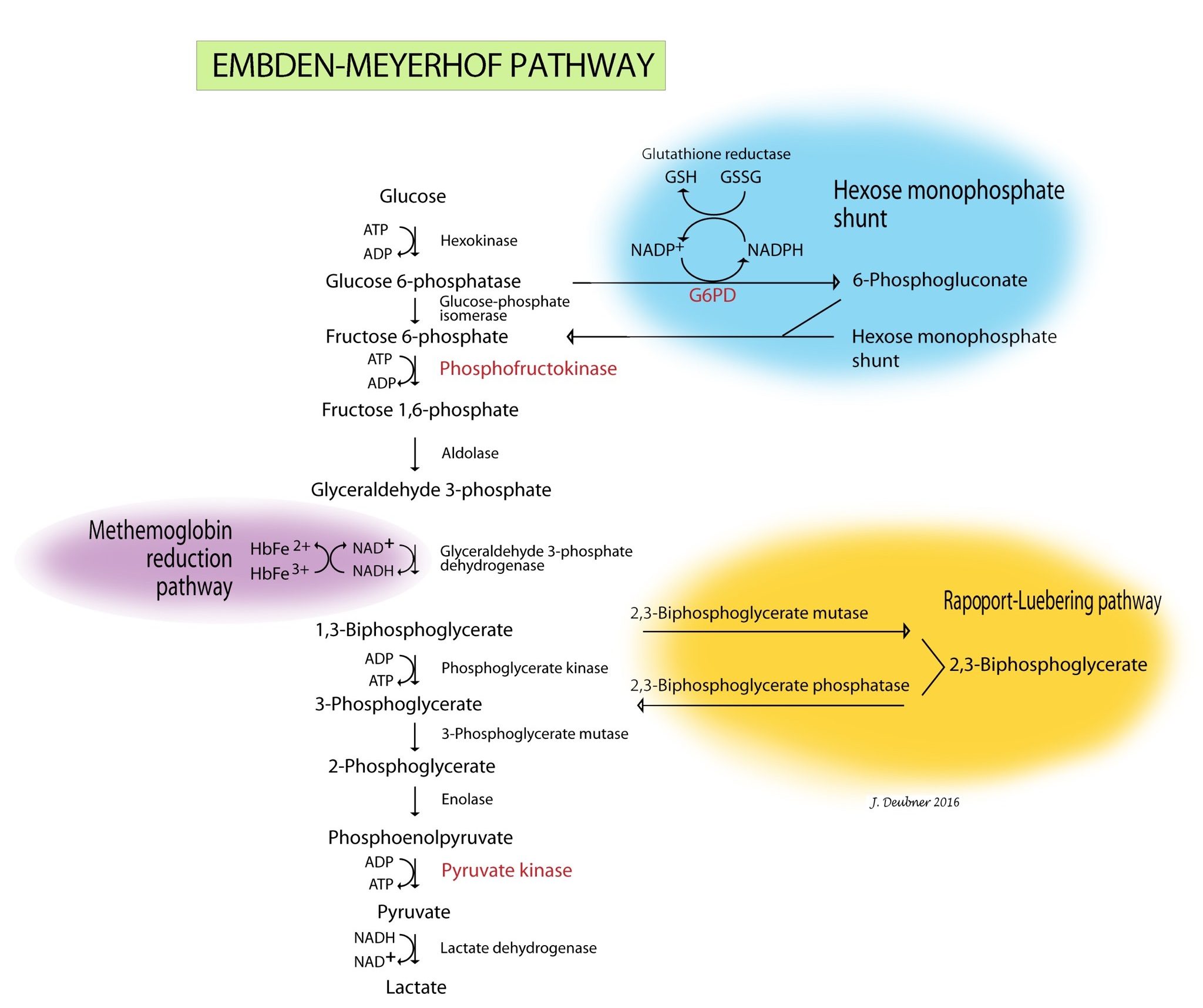
When reticulocytes mature and lose their mitochondria, they are no longer capable of oxidative metabolism. Mature erythrocytes derive their energy from ATP generated by anaerobic glycolysis (Embden-Meyerhof pathway) (Fig. 1.31). ATP provides the energy to maintain membrane ion pumps, cell shape, and deformability. Defects in this pathway lead to an inability to maintain normal water and electrolyte homeostasis within the red cells and to maintain normal shape and deformability. Intravascular hemolysis, premature destruction by the MPS in the spleen, or both may result from the lack of ATP.
Oxygen Affinity
A branch of the Embden-Meyerhof pathway, known as the Rapoport-Luebering pathway, is responsible for production of 2,3-biphosphoglycerate (2,3-BPG). 2,3-BPG levels affect the affinity that hemoglobin has for oxygen. Levels vary in different parts of the body and under different circumstances. For example, working muscles have a higher temperature and lower pH, which results in increased 2,3-BPG production such that hemoglobin releases oxygen more readily. In the lung, where acid is blown off and the temperature is cooler, less 2,3-BPG is produced and hemoglobin affinity for oxygen is increased. With chronic anemia, 2,3-BPG levels are increased so that oxygen is released to tissues more readily. Enzymopathy resulting in reduced synthesis of 2,3-BPG has not been reported in veterinary medicine, but would be expected to result in increased affinity of hemoglobin for oxygen, decreased release of oxygen to tissues and, ultimately, secondary erythrocytosis.
Prevention of Oxidative Damage
Erythrocytes must be capable of preventing chemical injury (oxidative damage) from the high concentrations of oxygen that they transport. This is accomplished through two other branches of the Embden-Meyerhof pathway, the hexose monophosphate shunt (also known as the pentose phosphate pathway) and the methemoglobin reduction pathway. Glucose-6-phosphate is the substrate for the hexose monophosphate shunt which maintains glutathione in the reduced state. Reduced glutathione is an intracellular buffer which protects red cells from oxidant injury, particularly by hydrogen peroxide and the superoxide anion, and also helps to stabilize the reactive sulfhydryl groups of hemoglobin. The methemoglobin reduction pathway returns oxidized hemoglobin (i.e. methemoglobin) to its reduced state (ferrous/Fe2+) which is capable of oxygen transport. Defects in the hexose monophosphate shunt lead to oxidative damage to erythrocytes, hemoglobin denaturation, and Heinz body hemolytic anemia. Errors in the methemoglobin reduction pathway prevent reduction of oxidized hemoglobin. Erythrocytes are unable to transport oxygen and cyanosis develops.
Cellular injury resulting from free radicals and other unstable molecules such as reactive oxygen species; identified by the presence of Heinz bodies and eccentrocytes.
Erythrocyte metabolic pathway responsible for production of ATP.
Erythrocyte rupture or destruction; may occur in vitro or in vivo as a pathologic process.
Erythrocyte metabolic pathway responsible for generation of 2,3-BPG.
2,3-bisphosphoglycerate; also called 2,3-DPG or 2,3-diphosphoglycerate. Molecule involved in regulating oxygen affinity of hemoglobin.
Increase in hematocrit (PCV) in peripheral blood; usually accompanied by increases in hemoglobin and RBC numbers.
Erythrocyte metabolic pathway responsible for providing protection against oxidants and oxidative damage.
Oxidized, non-functional hemoglobin.
Enzyme involved in protection of erythrocytes from oxidative damage.
Erythrocyte metabolic pathway responsible for maintaining hemoglobin in a reduced (functional) state.
Aggregate of denatured hemoglobin within a RBC, caused by oxidative damage.
Anemia due to destruction of RBCs. Lysis may be intravascular (within blood vessels) or extravascular (within tissues rich in mononuclear phagocytes such as spleen and liver), or both.

