Chloride
The source of Cl– is the diet, usually together with Na+. Regulation of Cl– is renal, both passive along with Na+ and active due to reabsorption in the loop of Henle. Cl– changes usually parallel Na+ changes: if one Na+ is lost, one Cl– will also be lost in order to maintain electroneutrality. Under certain circumstances, Cl– will change inversely to bicarbonate (HCO3–) and, therefore, not in parallel with Na+ (e.g. one Cl– gained and one HCO3– lost).
Hyperchloremia
A relative increase in Cl–, paralleling Na+, is seen with ECF volume depletion, as described in the section on Na+. Hyperchloremia can also occur with decreased excretion of Cl– due to proximal renal tubular acidosis, a dysfunction in HCO3– resorption that results in HCO3– loss in the urine. In this situation, Cl– is retained to maintain electroneutrality. Hyperchloremic metabolic acidosis with a normal anion gap will be seen on a biochemical panel.
Hypochloremia
A relative decrease in Cl–, paralleling Na+, occurs with increased ECF from overhydration. Anorexia, diarrhea, or inappetence, particularly in herbivores, will result in hypochloremia that is expected to be proportional to the magnitude of hyponatremia.
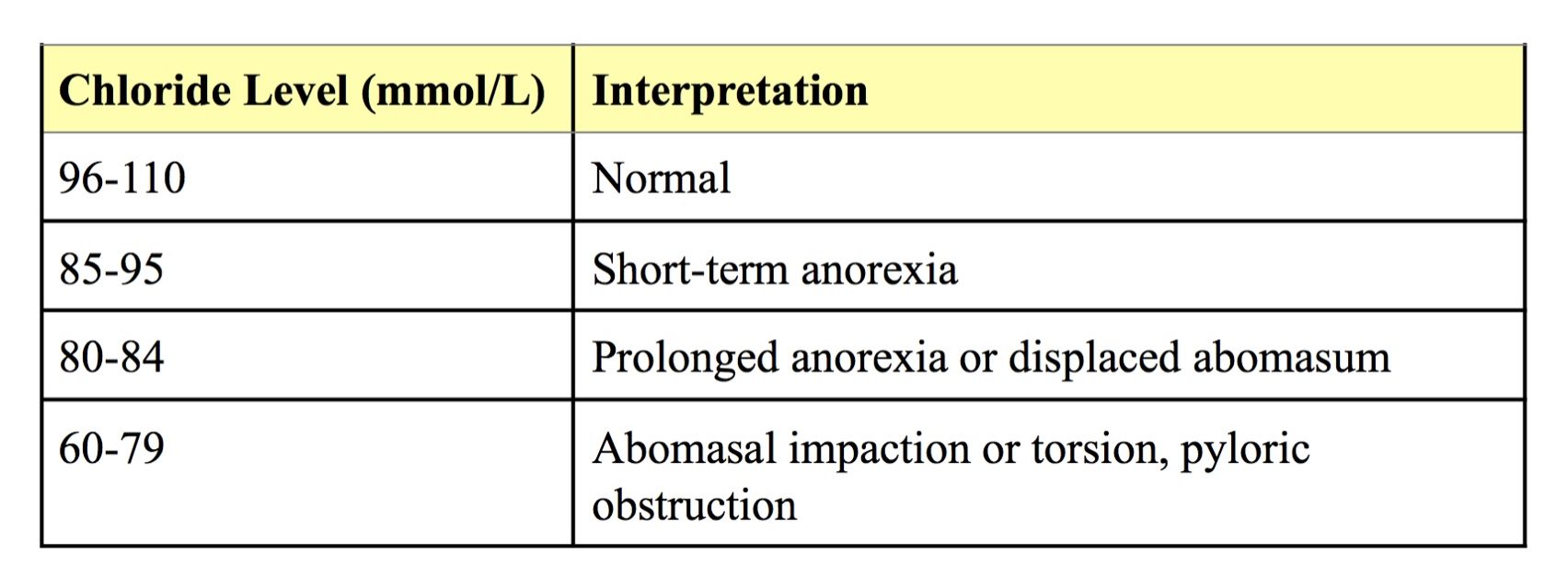
Hypochloremia that is greater than the degree of hyponatremia is commonly seen in ruminants with abomasal disorders, such as stasis, displacement and torsion, in which Cl– is sequestered in the abomasum (Table 6.2). Selective loss of Cl– is seen in monogastric animals with persistent high intestinal vomiting or stasis; causes include foreign body, tumor mass, gastrinoma (rare) and obstruction due to edema as can occur with pancreatitis in dogs.
The principal anion found in both equine sweat and saliva is Cl–, which explains Cl– losses when sweating and salivating are excessive. Esophageal obstruction (choke) is an important mechanism of marked salivary loss of Cl– in the horse. Note that this is not the case in ruminants as HCO3– is the predominant anion in saliva of these species.
Difference between unmeasured anion and cation concentrations, calculated using the formula: (Na+ + K+) minus (Cl- + HCO3-.)

