Erythropoiesis
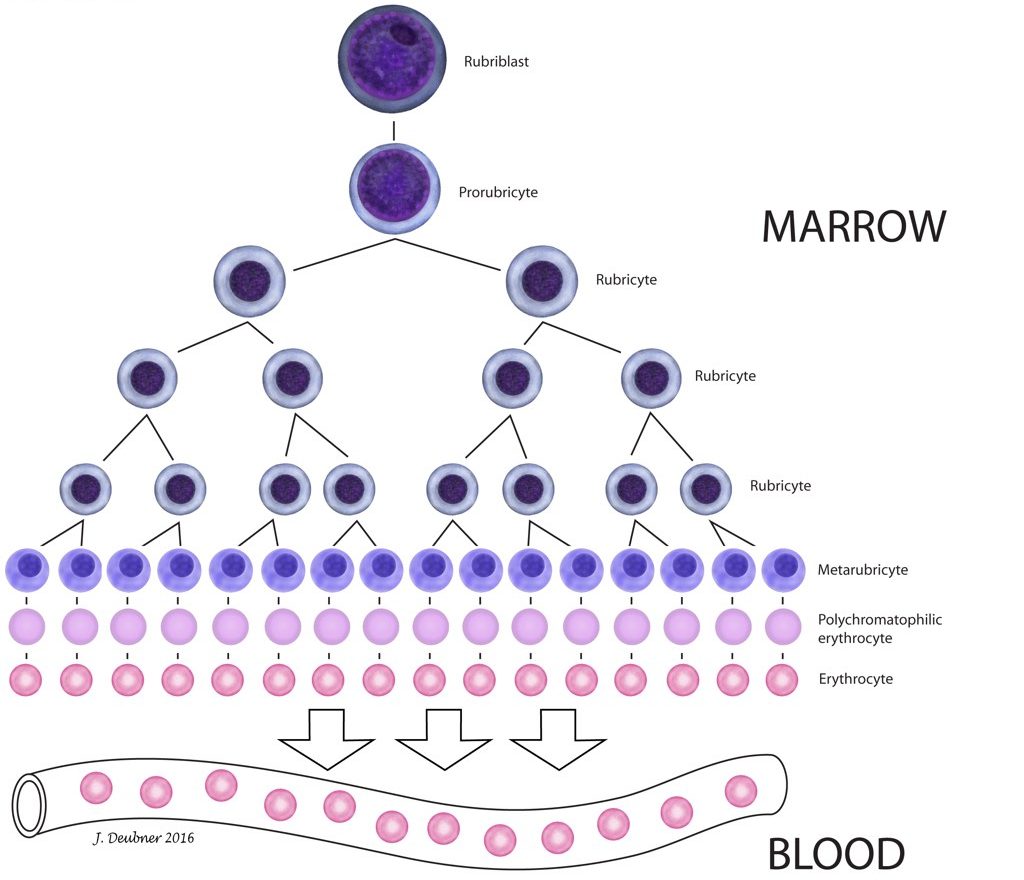
The production of erythrocytes from hemopoietic stem cell to mature circulating RBC is known as erythropoiesis. Erythrocytes deliver oxygen to tissues, remove carbon dioxide from tissues, and buffer acid-base changes in the circulation. Tissue oxygenation is the main regulator of RBC production, and conditions associated with tissue hypoxia stimulate the bone marrow to increase RBC production. When tissue oxygenation is adequate, total erythrocyte mass fluctuates very little as a steady state exists between RBC production and RBC loss. Erythropoietin (EPO) is the most important growth factor for maintaining erythroid proliferation. The committed erythroid precursor, which immediately follows the erythrocyte/megakaryocyte progenitor (Fig. 1.1), undergoes up to 5 mitotic divisions over 5 days. The earliest recognizable erythroid precursor is the rubriblast, followed by differentiation sequentially to the prorubricyte, rubricyte, metarubricyte, polychromatophilic erythrocyte, and the mature erythrocyte (Fig. 1.2). Progression of erythropoiesis is accompanied by a decrease in EPO receptors and an increase in transferrin receptors on the surface of maturing erythrocytes. Transferrin receptors allow incorporation of iron into erythrocytes for hemoglobin synthesis. Hemoglobin comprises 4 globin chains, each bound to a heme molecule containing iron. Hemoglobinization of the red cell cytoplasm is most active during the rubricyte stage. Also, cell division stops following the rubricyte stage as hemoglobinization nears completion and the nucleus condenses. At the end of the metarubricyte stage, the pyknotic nucleus is extruded and phagocytized by local macrophages, usually within the bone marrow.
Although nucleated erythrocytes are not usually found in the peripheral blood, a low percentage of circulating polychromatophilic cells are present under normal circumstances in most species (e.g. about 1% in the dog). The horse is an exception in that immature erythrocytes are rarely released into the peripheral blood in this species, even when intense erythroid hyperplasia is occurring in the bone marrow in response to anemia. In other common species, residual ribosomes and RNA, reflecting the end of protein synthesis (mainly hemoglobin), are responsible for the purplish-blue colouration of polychromatophilic erythrocytes with Romanowsky-type stains, such as Wright-Giemsa (Fig. 1.3). Polychromatophilic erythrocytes lose their residual RNA within 24-48 hours of release from the bone marrow to become mature circulating RBCs. When there is increased polychromasia on smears stained with Romanowsky stains, an aliquot of the blood may be mixed with new methylene blue (NMB) stain and smears made, in order to enumerate the immature RBCs. The cells containing clumps or aggregates of RNA, that has been precipitated by the NMB, are called reticulocytes. The reticulocyte count parallels the degree of polychromasia seen with Romanowsky stains, but is more accurate as at least 1000 RBCs are counted to determine the percent that are reticulocytes (Fig. 1.4).
Part of hemopoiesis dealing with the production of erythrocytes from stem cells to mature circulating red blood cells.
All the erythroid cells in the body, including precursors
Growth factor that maintains erythroid proliferation.
Polychromatophil; an anucleate (in mammalian species), immature erythrocyte containing cytoplasmic RNA and ribosomes which give the cell a bluish-pink appearance with Romanowsky stains (e.g. Wright-Giemsa).
Oxygen-carrying molecule within erythrocytes.
Condensed or shrunken, as in the nucleus of a metarubricyte or apoptotic cell.
Erythrocyte precursor; often used to refer to metarubricytes in peripheral blood.
Increased numbers of erythrocyte precursors seen on examination of bone marrow, e.g. in response to anemia.
Decrease in hematocrit (PCV) recognized on the complete blood count (CBC); usually hemoglobin concentration and RBC numbers are also decreased.
Increased numbers of anucleate, immature erythrocytes that stain bluish-pink with Romanowsky stains due to the presence of cytoplasmic RNA.
Routine stain for blood smears.
Anucleate (in mammalian species), immature erythrocyte containing cytoplasmic RNA and ribosomes which are precipitated by staining with new methylene blue.

