Indicators of Cholestasis
Alkaline phosphatase (ALP)
ALP is present in many tissues in the body; however, increased serum ALP activity is usually due to bile stasis or increased osteoblast activity. With bile stasis, ALP synthesis is increased in hepatocytes and bile duct epithelium. Also, existing ALP within cell membranes may be solubilized and released into the peripheral blood. In the dog, ALP can also be induced by various drugs, particularly anticonvulsants and corticosteroids, and increased concentrations of endogenous corticosteroids (e.g. with chronic stress/illness or hyperadrenocorticism). The serum half-life of ALP is about 72 hours in the dog and 6 hours in the cat. The serum activity of ALP is not routinely measured in large animals since GGT is a more reliable indicator of cholestasis in these species.
Although it is possible to differentiate hepatic and corticosteroid isoforms of ALP activity in the dog, this information is seldom useful since many disorders are associated with increased activities of both. For instance, with corticosteroid administration in dogs, hepatic isoform ALP activity increases initially and corticosteroid isoform ALP activity increases more gradually. Also, with hepatobiliary disease, stress associated with the illness can result in corticosteroid isoform ALP increases along with hepatic isoform ALP increase due to cholestasis. Increased bone isoform ALP activity is seen in young, growing animals, in feline hyperthyroidism (sources are both bone and liver), and with skeletal lesions involving extensive bone lysis, remodelling, or both. Breed related increases in serum ALP activity are reported in certain Scottish Terrier and Siberian Husky dogs. The increase has been determined to be the bone isoform in Siberian Husky puppies evaluated. In the Scottish Terrier dogs, isoform data is unavailable.
Marked elevations in serum ALP activity occur with hepatic lipidosis in cats. These cats are usually icteric as well. Considerable hepatocyte enlargement results from intracellular lipid accumulation in this disease. Canalicular cholestasis occurs which leads to both clinical icterus and increased ALP synthesis and release. The other main enzymatic indicator of cholestasis, GGT, is not released or synthesized to the same degree as ALP, a feature which can be useful in differentiating hepatic lipidosis from other cholestatic liver diseases in cats. The reason for the different magnitude increase of ALP activity compared to GGT activity in these cats is not fully understood, but may relate to their cellular locations. GGT is primarily associated with biliary epithelium, whereas ALP is primarily associated with hepatocyte membranes, particularly those adjacent to canalicular surfaces. With hepatic lipidosis the lipid accumulation occurs within hepatocyte cytoplasms, causing impingement on canalicular bile flow.
Gamma-glutamyltransferase (GGT)
Although GGT is synthesized by most tissues, serum GGT is mainly from the liver. Cholestasis results in both increased synthesis of this enzyme as well as increased release from cell membranes. Serum GGT activity may be increased in dogs receiving corticosteroids (although to a lesser degree than ALP); these increases may be due to increased production or secondary to steroid hepatopathy resulting from corticosteroid therapy. Although both ALP and GGT are used as indicators of cholestasis in dogs and cats, GGT alone is used in horses and ruminants. Nursing neonates in many species, particularly dogs, sheep, and cattle, have increased serum GGT activity due to absorption of GGT in colostrum.
Bilirubin
Hemoglobin degradation is the major source of bilirubin (Fig. 8.1). Senescent erythrocytes are phagocytized by macrophages and degraded to globin and heme. The iron in heme is efficiently recycled for erythropoiesis; the protoporphyrin in heme is degraded to bilirubin. Bilirubin is released from macrophages and transported to the liver bound to protein, usually albumin. This bilirubin is insoluble, unconjugated, and, for the most part, unfilterable by the kidneys. At the hepatocyte membrane, bilirubin is released from its carrier protein and transported into the hepatocyte cytosol where conjugation to sugar groups, particularly glucuronic acid, occurs. Bilirubin glucuronide is released into bile canaliculi and flows out through the biliary tract into the intestines. This conjugated bilirubin is water soluble, mainly non-protein bound, and excretable by the kidneys. A small amount of conjugated bilirubin is protein bound (known as delta bilirubin or biliprotein), and this remains in the blood for a longer period of time. The conjugated bilirubin excreted in bile is converted to urobilinogen by local bacteria in the intestinal tract. Most is then excreted as stercobilinogen in the feces; however, a small amount of urobilinogen is reabsorbed and either re-excreted by the liver or excreted in the urine. In theory, the presence of urobilinogen in the urine rules out complete biliary obstruction. Because biliary tract blockages are often incomplete and because urobilinogen may or may not be present in the urine of healthy animals, the diagnostic utility of measuring urine urobilinogen is questionable and many veterinary laboratories no longer report this result.
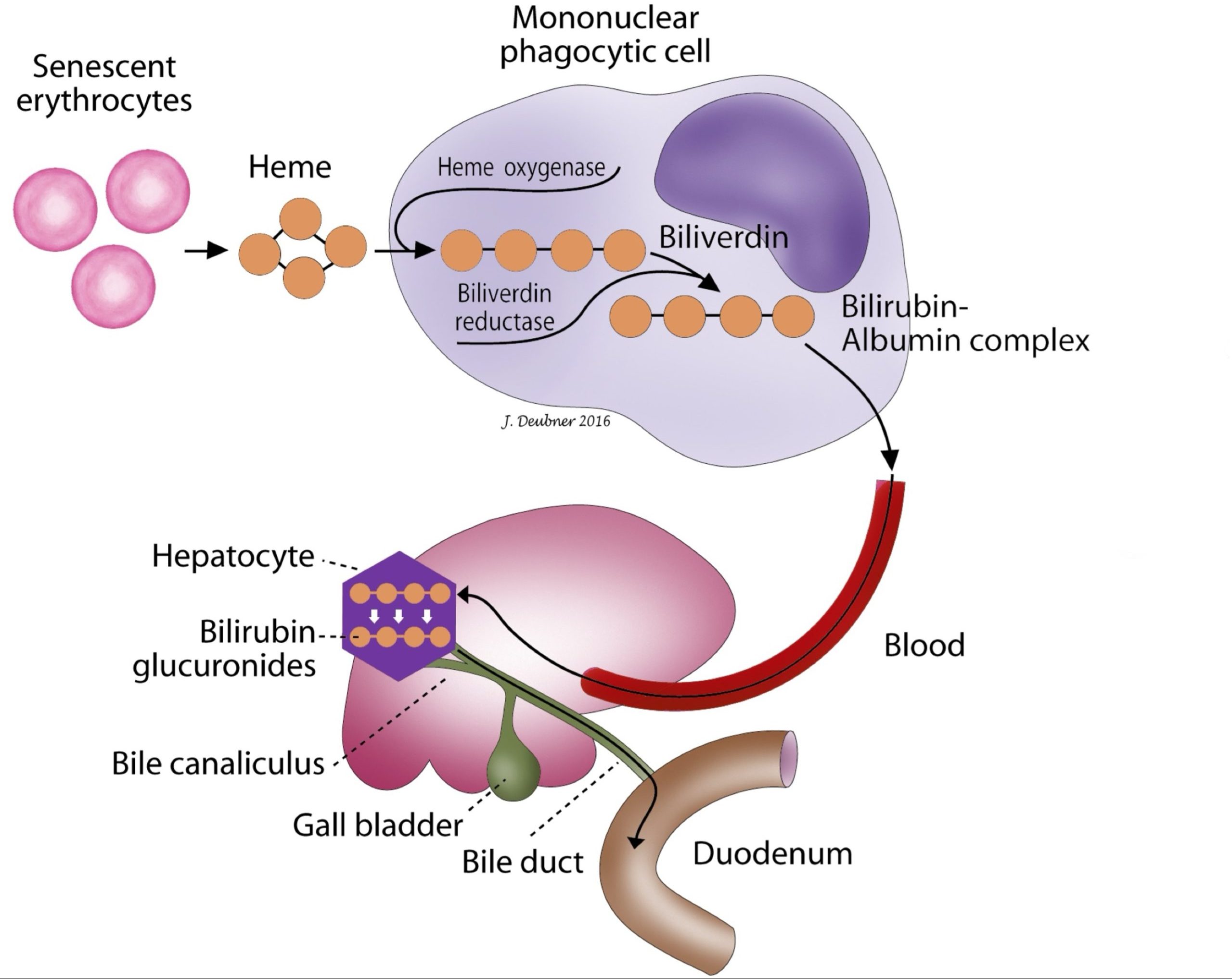
Hyperbilirubinemia may be seen with hepatocellular pathology such as intracytoplasmic lipid accumulation, cholangitis or cholangiohepatitis, bile duct occlusion due to tumor growth(s), or obstruction with calculi and parasites. The term “cholestasis” refers to interruption or obstruction of bile flow or excretion of bile. Cholestasis may be intrahepatic from hepatic pathology causing compression of bile canaliculi, or from periportal lesions such as cholangitis. Alternatively, cholestasis may be extrahepatic when physical obstruction of bile flow results from pathology involving the biliary tree, such as neoplasia, pancreatitis, or cholelithiasis, which is usually distal to the hepatic parenchyma.
Hyperbilirubinemia without liver disease may also occur in the absence of increased RBC breakdown. In such cases, hyperbilirubinemia may result when one or more steps in bilirubin metabolism and excretion are compromised. These steps include: delivery of bilirubin to the liver; uptake and conjugation by hepatocytes; excretion into the biliary tract; and bile flow through the distal biliary tract and gall bladder (except for horses) into the intestinal tract. For example, anorexia or starvation can result in impaired hepatic blood flow with decreased hepatocyte uptake of unconjugated bilirubin, decreased conjugation of bilirubin, or both. Similarly, endotoxemia, septicemia, and shock can impair hepatic blood flow resulting in hyperbilirubinemia. In addition, sepsis may result in functional cholestasis due to increased concentrations of cytokines such as TNFα and IL-6 which impair excretion of conjugated bilirubin from hepatocytes. Anorexic horses may have mild hyperbilirubinemia due to release of free fatty acids (FFA) from stored fat; these FFA are transported to the liver and compete with bilirubin for the same receptor on the hepatocyte membrane, resulting in increased unconjugated bilirubin in the blood.
Differentiating conjugated from unconjugated bilirubin in hyperbilirubinemic states often does not help differentiate the various causes of icterus. The importance of history, physical findings, results of ancillary tests, and concurrent laboratory abnormalities cannot be underestimated in determining the etiology of hyperbilirubinemia.
Important species differences exist with respect to bilirubin metabolism. The renal threshold for conjugated bilirubin is low in most domestic animals, but especially dogs. Also, in dogs, renal tubular epithelial cells are capable of bilirubin conjugation during hemoglobin catabolism by these cells; this process appears to be greater in male dogs than female dogs. Therefore, small amounts of bilirubin are commonly observed in urine from normal dogs without the presence of hyperbilirubinemia. In most other species, bilirubinuria accompanies or may even precede hyperbilirubinemia.
Common endocrine disease of older cats associated with thyroid gland hyperplasia or a functioning thyroid adenoma.
General term for fat, including triglycerides, phospholipids, cholesterol.
Jaundice; deposition of pigment in skin, mucous membranes and sclera due to hyperbilirubinemia; usually results in a yellow discoloration of skin and mucous membranes.
Break-down product of hemoglobin.
Oxygen-carrying molecule within erythrocytes.
Red blood cell (RBC); an anucleate (in mammalian species) cell containing hemoglobin needed for oxygen transport. Typically shaped like a bi-concave disk.
Mononuclear phagocyte found in tissues that develops from circulating blood monocytes and fulfills many roles in normal immune function including antigen presentation.
Part of hemopoiesis dealing with the production of erythrocytes from stem cells to mature circulating red blood cells.
Most abundant plasma protein in health; maintains oncotic pressure.
Insoluble form of bilirubin that is not filtered by the kidneys. Reported as indirect bilirubin on biochemical panels.
Also called biliprotein; conjugated bilirubin that is protein bound and thus persists in the blood for a longer time
Abnormal uncontrolled growth of cells that are unresponsive to normal physiologic growth controls; may be benign or malignant.
Referring to cells of the skin and adnexa, lining of the airways, intestines, and urinary tract, renal tubules, liver, and glandular tissues.

