Anion Gap
Anion gap (AG) is useful primarily for assessing metabolic acidosis. The concept is introduced here but more information is below in the section on metabolic acidosis. AG is the difference between the unmeasured anion and cation concentrations (Fig.6.2). It is not a true gap or difference because, by the law of electroneutrality, the sum of the concentrations of all negatively charged particles (anions) in the extracellular/intravascular fluid must equal the sum of the concentrations of all positively charged particles (cations) in the extracellular/intravascular fluid.
AG is a calculated value that appears on the biochemical panels (or can be calculated manually) using the following formula:
AG = (Na+ + K+) – (Cl– + HCO3–)
The cations used in the formula (Na+, K+) and the anions used in the formula (Cl–, HCO3–) are referred to below as the measured cations and anions, respectively. There are other cations and anions that are measured in routine biochemical panels and these are referred to as “unmeasured” with regards to anion gap discussions. The measured cations (Na+ and K+) account for about 95% of the total serum cations and the measured anions (Cl– and HCO3–) account for about 85% of the total serum anions (see Table 6.1). “Unmeasured” or other anions are proteins; organic anions, such as lactic acid, pyruvic acid, and ketoacids; inorganic anions, such as phosphate and sulfate; and exogenous anions, such as ethylene glycol, salicylates, methanol, and paraldehyde. “Unmeasured” or other cations are Mg++ and Ca++.
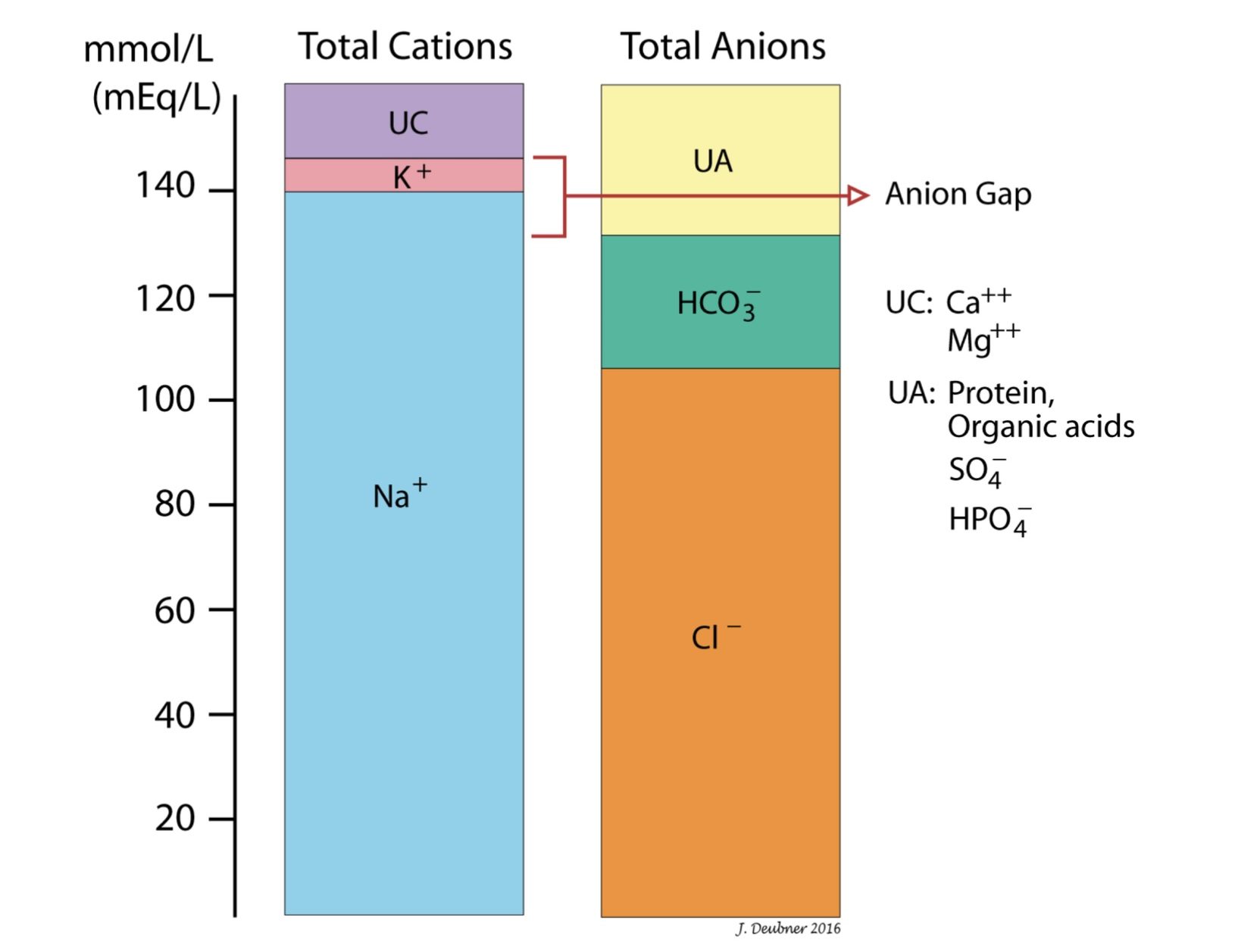
TOTAL CATIONS = TOTAL ANIONS
Na+ + K+ + “unmeasured” cations = Cl– + HCO3– + “unmeasured” anions
This equation can be rearranged as follows:
(Na+ + K+) – (Cl– + HCO3–) = “unmeasured” anions – “unmeasured” cations = the difference between “unmeasured” anions and “unmeasured” cations = Anion Gap
An increased AG is usually due to increased unmeasured anions and reflects metabolic acidosis (see below)(Fig.6.3). For example, with lactic acidosis, the acid dissociates into H+ and its anion, lactate-. The H+ is titrated by HCO3–, forming carbonic acid (H2CO3) which dissociates into CO2 and water, leaving its anion behind to be excreted with a cation, such as Na+ or K+. Thus, HCO3– is decreased in the serum and lactate- is the “footprint” of the acid gain. In this situation, there is a high anion gap metabolic acidosis which is referred to as an acid gain or titration type of metabolic acidosis.
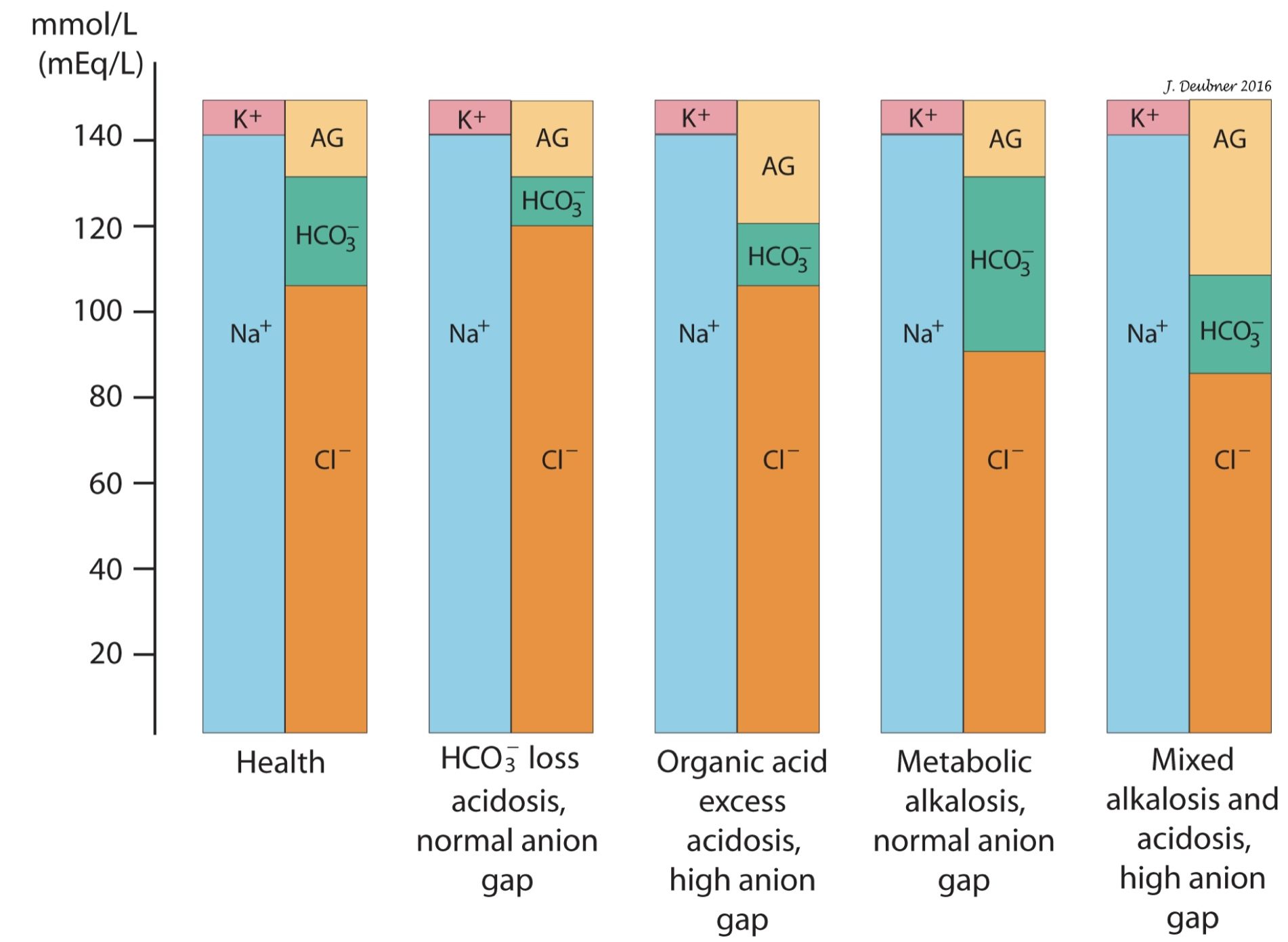
A decreased AG is usually due to decreased unmeasured anions such as albumin, or, less commonly, increased unmeasured cations. For every 10 g/L that albumin is decreased from normal, there is a decrease in anion gap of 4 mmol/L. Note that a decreased anion gap does not have any clinical significance and does not indicate the presence of a metabolic alkalosis.
Ions with a positive charge.
Most abundant plasma protein in health; maintains oncotic pressure.

