Lymphocytes
Lymphopoiesis
The hemopoietic stem cell differentiates along one of two lines into the common myeloid progenitor (CMP) and the common lymphoid progenitor (CLP). The heterogeneous family of lymphocytes differentiates from the CLP (Fig. 1.1).
B lymphocytes proliferate to the pre-B cell stage in the bone marrow under the influence of IL-7. Differentiation to mature B cells occurs in peripheral lymphoid tissues and parallels surface expression of immunoglobulin under the influence of IL-2, IL-4, and IL-5. Terminal differentiation into plasma cells occurs under the influence of IL-6, IL-4, and IL-10 resulting in cells that are morphologically distinct and able to secrete antibody. Typical plasma cells have eccentrically placed nuclei, royal blue cytoplasms, and prominent perinuclear clear zones (Golgi zones) Fig. 2.11.
T lymphocyte development involves migration of the CLP to the thymus. Antibodies to cell surface markers known as cluster of differentiation (CD) antigens, along with the form of T cell receptor expressed, identify the subtype of T cell. Although most T cell development occurs as cells migrate through the thymus from cortex to medulla, final differentiation of cytotoxic and helper T cells occurs in the peripheral blood, lymph nodes, and spleen. T cell differentiation requires IL-7, IL-1, IL-2, and IL-4.
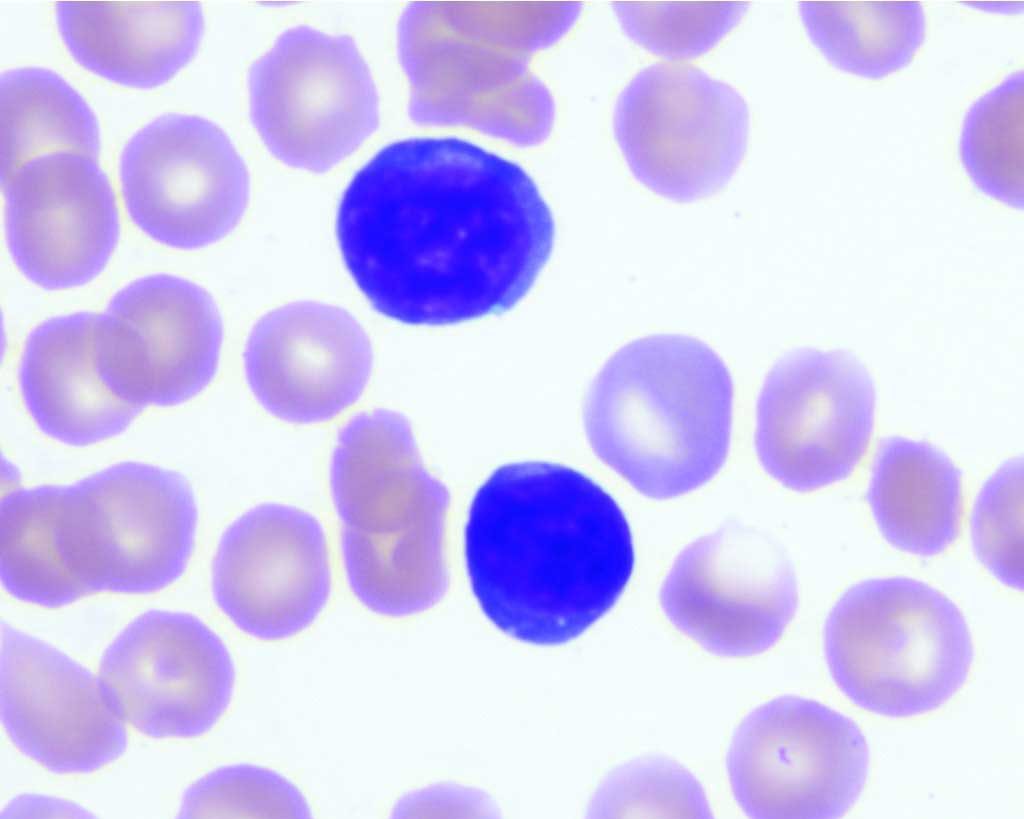
Lymphoblasts are large lymphocytes with high nuclear to cytoplasmic ratios, prominent nucleoli, and darkly stained nuclei and cytoplasms (see Fig. 3.3). They are not usually present in the peripheral blood. As lymphocytes mature, they become increasingly smaller, nucleoli disappear, and the cytoplasmic staining is variable, from pale to dark blue. Peripheral blood lymphocytes are often small mononuclear cells with a high nuclear to cytoplasmic ratio and darkly stained chromatin. Only a small rim of cytoplasm may be visible beyond the borders of the nucleus.
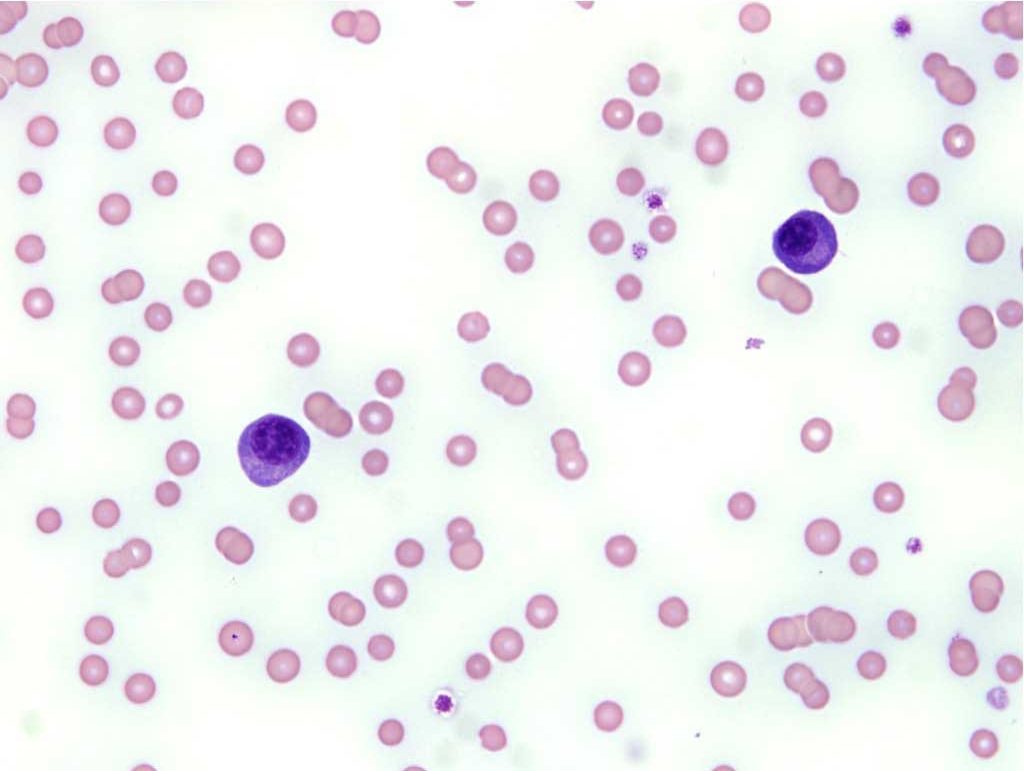
T cells predominate in the peripheral blood but usually cannot be differentiated from B cells on routine blood smear examination. Exceptions are the presence of large magenta cytoplasmic granules indicating cytotoxic T cell or natural killer cell origin (see Fig. 3.5), and differentiation to plasma cells indicating B cell lineage. Plasma cells are uncommon in the peripheral blood and could indicate immunologic stimulation or plasma cell neoplasia. See Fig. 2.12 for lymphocytes from the common domestic species.
The predominant leukocyte in bovine blood is the lymphocyte and two forms are seen. In addition to the small lymphocyte, cattle also have larger lymphocytes with abundant pale staining cytoplasms. Small pink granules are common in bovine lymphocytes. In any species, larger lymphocytes with very darkly stained nuclei and cytoplasms are occasionally seen. These are interpreted to be immunologically stimulated lymphocytes (immunocytes), also called reactive lymphocytes (Fig. 2.13).
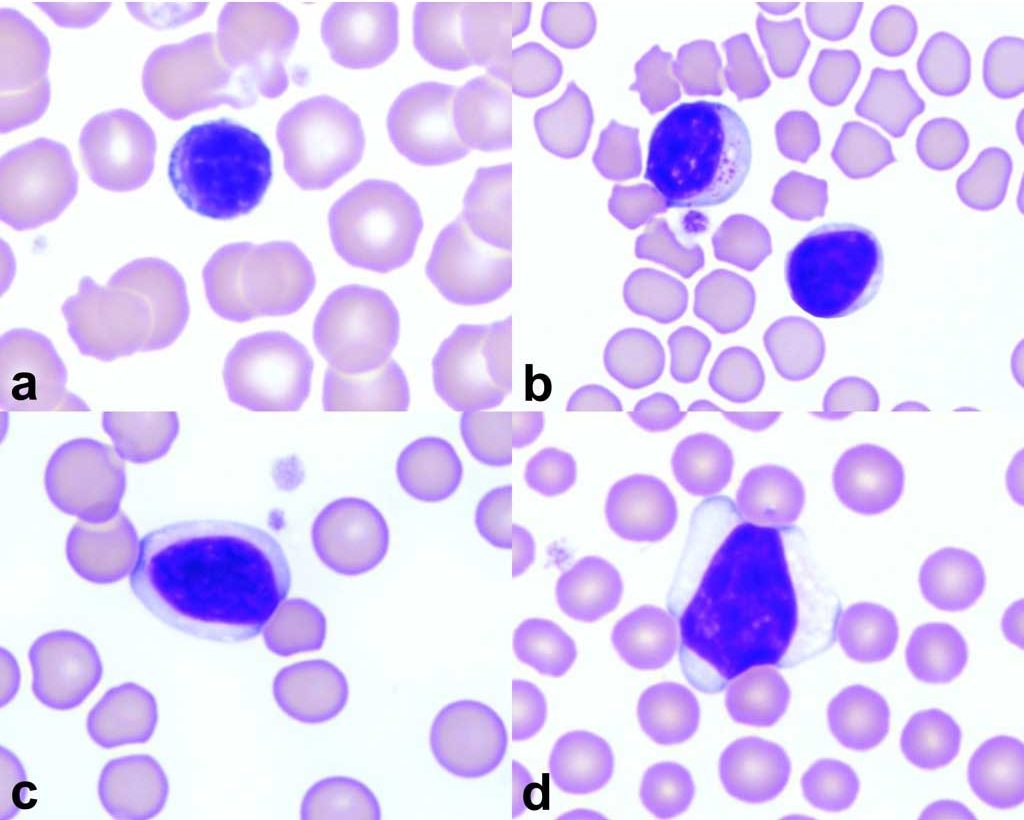
Lymphocytosis
Elevations in peripheral blood lymphocyte numbers can occur with chronic antigenic stimulation due to long-standing exposure to bacterial, viral, or rickettsial agents. Young animals exposed to a multitude of antigens for the first time, particularly associated with vaccination, may respond with lymphocytosis. Acute epinephrine release, as seen with extreme excitement or fear especially in cats, but also in horses, can result in lymphocytosis. Endogenous corticosteroid deficiency from hypoadrenocorticism results in a lack of stress lymphopenia or an absolute lymphocytosis in an ill or stressed animal (usually dog). Finally, unexplained lymphocytosis and/or abnormal lymphocyte morphology should trigger investigation for neoplastic disease.
Lymphopenia
Lymphopenia is very common in veterinary medicine, probably because most of our CBCs are performed on ill/stressed animals that are likely secreting excess cortisol. Routine CBCs on clinically healthy animals are done less commonly. In addition to “stress”, lymphopenia can be associated with a failure of lymphopoiesis, or increased lymphocyte destruction, as with hereditary or acquired immunodeficiency syndromes. Also, accelerated lymphocyte loss can occur with leakage of chyle, particularly when a chylous effusion is repeatedly drained.
Pluripotential cell that gives rise to all hemopoietic cell lines; also capable of self-renewal.
Lymphocyte involved in humoral immunity; may differentiate into a plasma cell
T lymphocyte subset involved in regulation of the specific immune response.
Large lymphocyte with prominent nucleolus; may be a lymphocyte precursor or neoplastic lymphocyte.
Comparison of the relative size of the nucleus and cytoplasm of a cell; a cell with abundant cytoplasm has a low N:C ratio, while a cell with a large nucleus and scant cytoplasm has a high N:C ratio. A high N:C ratio may be seen in neoplastic cells, but also in some normal cells (e.g. small lymphocyte).
T lymphocyte subset involved in killing of virus-infected and neoplastic cells
Cytotoxic lymphocyte important in the non-specific immune response
See reactive lymphocyte
Immunologically stimulated lymphocyte that typically has darkly staining cytoplasm
Part of hemopoiesis involving the production of lymphocytes
Fluid accumulation due to the leakage of chyle; contains primarily small lymphocytes and triglycerides

