6.1. (Very) Brief Refresher of the Basics
A quick reminder of some of the material you will have encountered before will save time and allow new material to build directly on familiar concepts. Section 6.1 should be a review. If it is not, consider skimming a chapter on acids and bases from a general chemistry textbook to ensure these concepts are well understood.
6.1.1. Brønsted-Lowry Acid-Base Reactions
Recall that, under the Brønsted-Lowry definitions, an acid is a proton (hydrogen cation) donor, and a base is a proton acceptor (Scheme 6.1). After donating the proton, the acid becomes a conjugate base. After accepting the proton, the base becomes a conjugate acid. Depending on the relative acidity of the acid and conjugate acid the reaction may be reversible or irreversible.
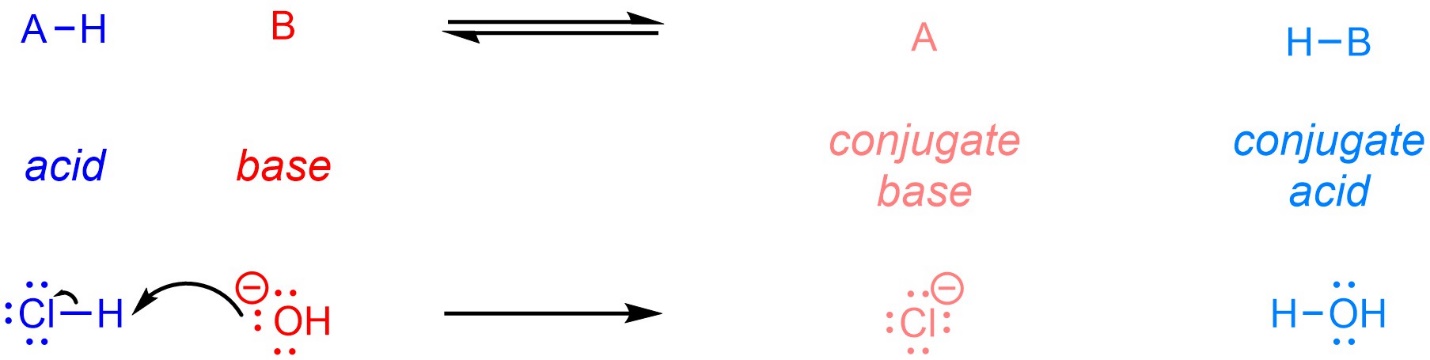
Scheme 6.1 – General Equation for and Example of a Brønsted-Lowry Acid-Base Reaction.
Generally, when describing acid-base reactions the compounds on the left of the arrow are called “acid” and “base” and those on the right are called “conjugate base” and “conjugate acid”. This is only a convention to allow readers to relate the compounds on each side to each other. It would be equally valid to drop “conjugate” from the labels and directly identify each compound as an acid or base.
6.1.2. Lewis Acid-Base Reactions
Recall that, under the Lewis definitions, an acid is an electron pair acceptor, and a base is an electron pair donor (Scheme 6.2). Protons are not the focus of Lewis acid-base reactions. Labelling compounds as Lewis acids and Lewis bases is contentious as the definition is very similar to another important concept in organic chemistry (see Section 7.2). Lewis acids often have incomplete octets and are often metals or metalloids, though these are not requirements for being a Lewis acid. Lewis bases have a lone pair (this is a requirement for being a Lewis base).
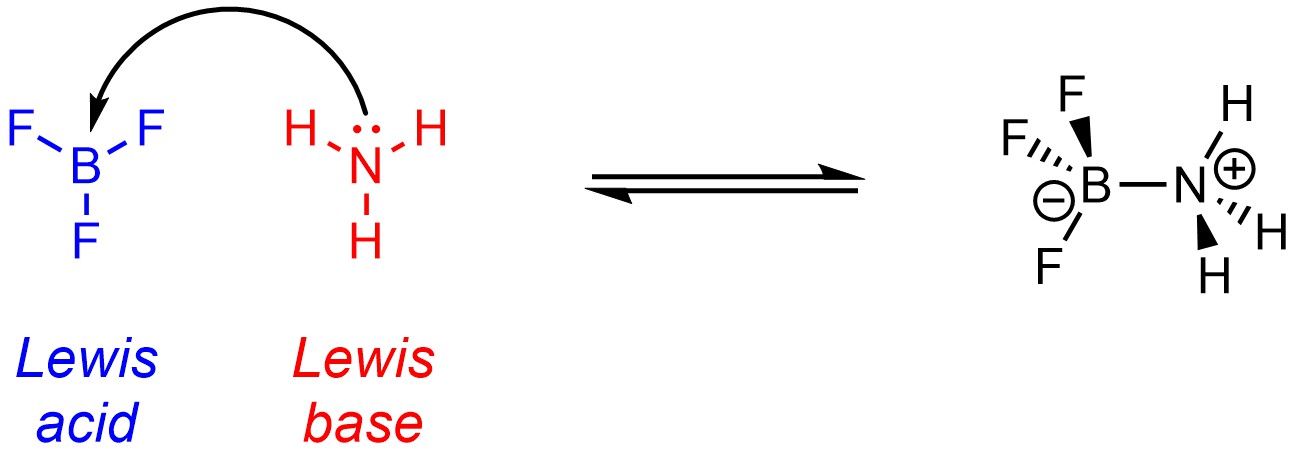
Scheme 6.2 – Example of a Lewis Acid-Base Reaction.
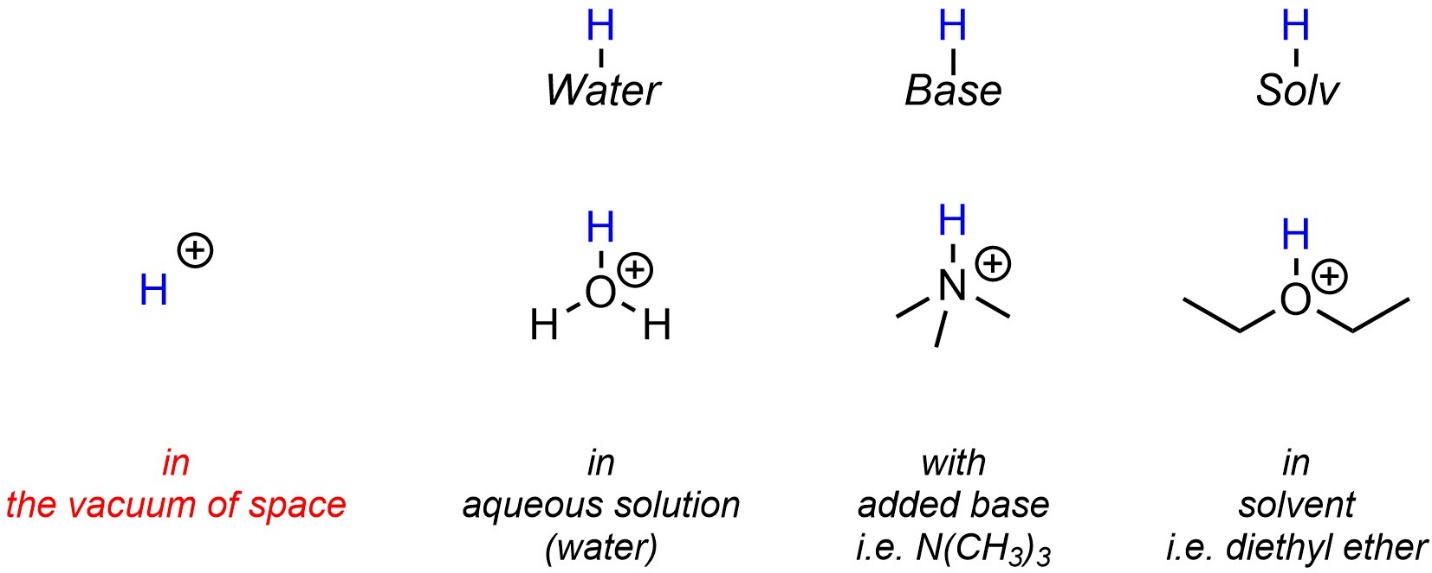
6.1.3. The “H+” Ion and Real Life
Single protons, “H+” cations, exist in the vacuum of space and in other exotic conditions. They do not exist under standard conditions. Instead, the proton associates with the most appropriate base in the medium it is in. Often this is water, an added base, or a solvent (Figure 6.1). Because it can be ambiguous or unclear what exactly the proton is associated with, many sources abbreviate this as “H+” when drawing reaction equations or mechanisms. However, if possible this notation should be avoided as it can lead to errors where a molecule (water, base, solvent) “disappears” at one step but is needed for a later step.