4.3. Unusual Cases of Chirality and Stereogenicity
Some special instances of chirality and stereogenicity are less common, but still important for an understanding of stereochemistry.
4.3.1. Stereocentres of Heteroatoms
Under the definition of stereogenic centre (see Section 4.2.2.3.) elements other than carbon can be stereogenic (Figure 4.24). This is slightly uncommon in nature but still plays important roles in some systems.
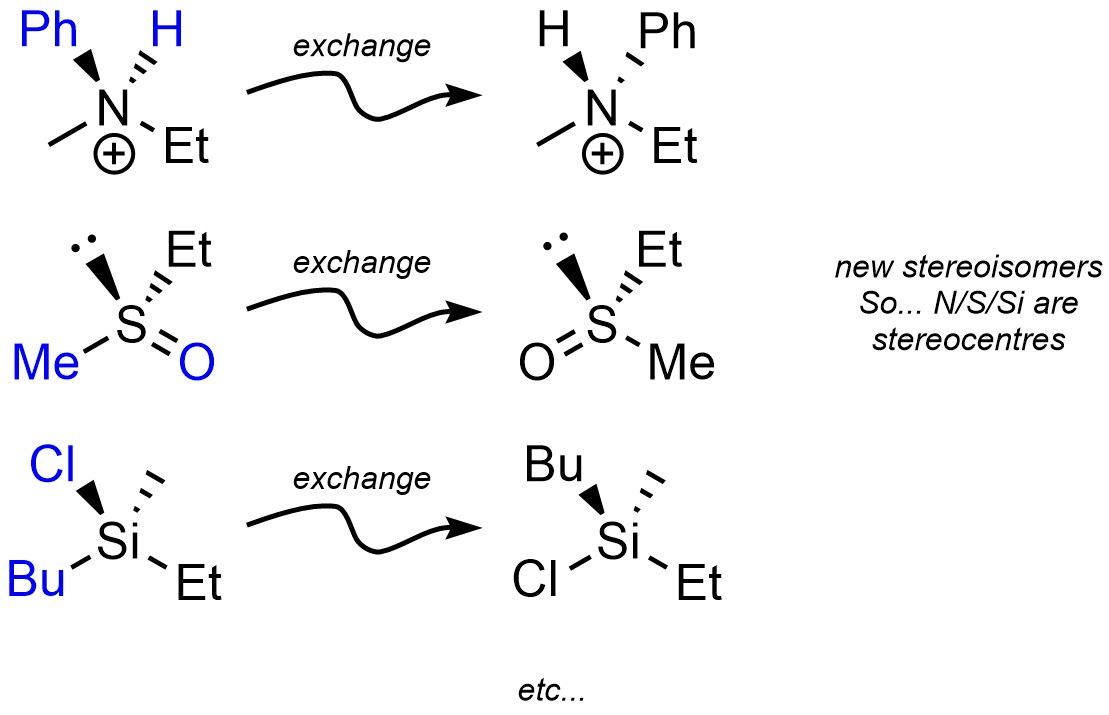
Figure 4.24 – Examples of Stereogenic Nitrogen, Sulfur, and Silicon.
Note that nitrogen atoms with a lone pair on them are not (usually) stereogenic. This is because the lone pair on the nitrogen is able to ‘move’ from one side to the other, causing the two isomers to interconvert (Figure 4.25). This process is sometimes referred to as (pyramidal) inversion. Why lone pairs on nitrogen are able to do this is beyond the scope of this text.
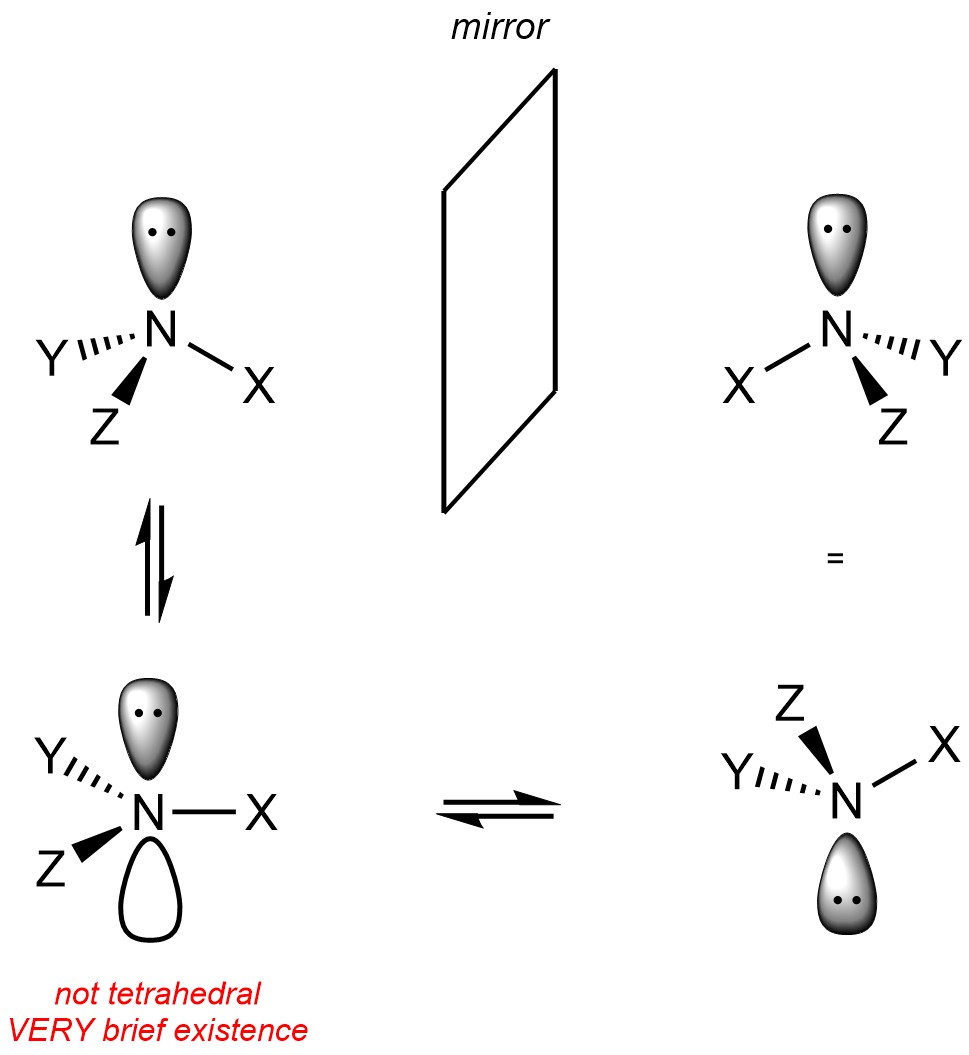
Figure 4.25 – Interconversion of Stereoisomers Due to Inversion at Nitrogen Atoms with Lone Pairs.
Nitrogen atoms with lone pairs can be stereogenic if they are part of highly rigid systems and thus cannot undergo inversion. Usually this requires a geometric constraint such as a ring system (Figure 4.26). These types of stereogenic centres are rare.
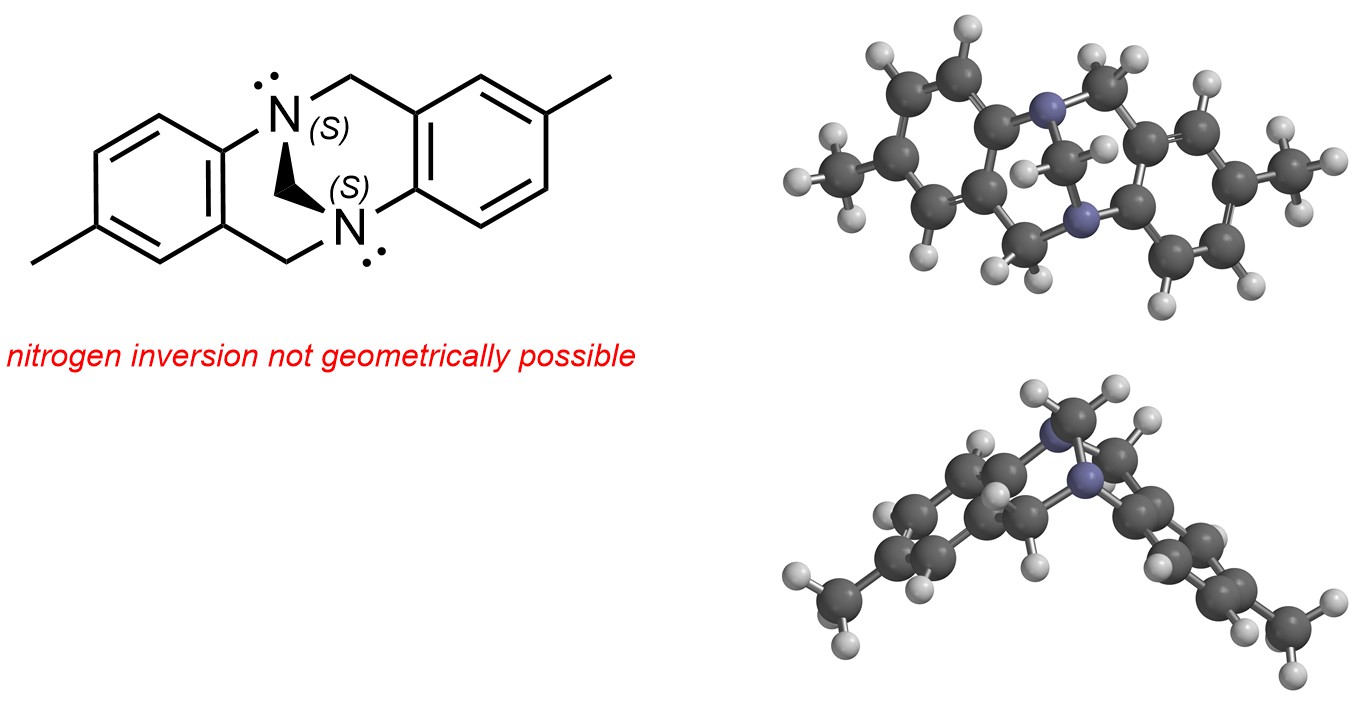
Figure 4.26 – Example of Stereogenic Nitrogen Atoms.
4.3.2. Advanced Stereocentres – Beyond Four Groups
Some older sources provide definitions of stereogenic centres that rely on “four different groups”. No part of the definition of stereogenic centres (see Section 4.2.2.3.) requires “four different groups”. Many commonly encountered stereogenic centres have this trait, but that is coincidental. For example, stereogenic centres with five or six groups are common in inorganic and organometallic chemistry (Figure 4.27).
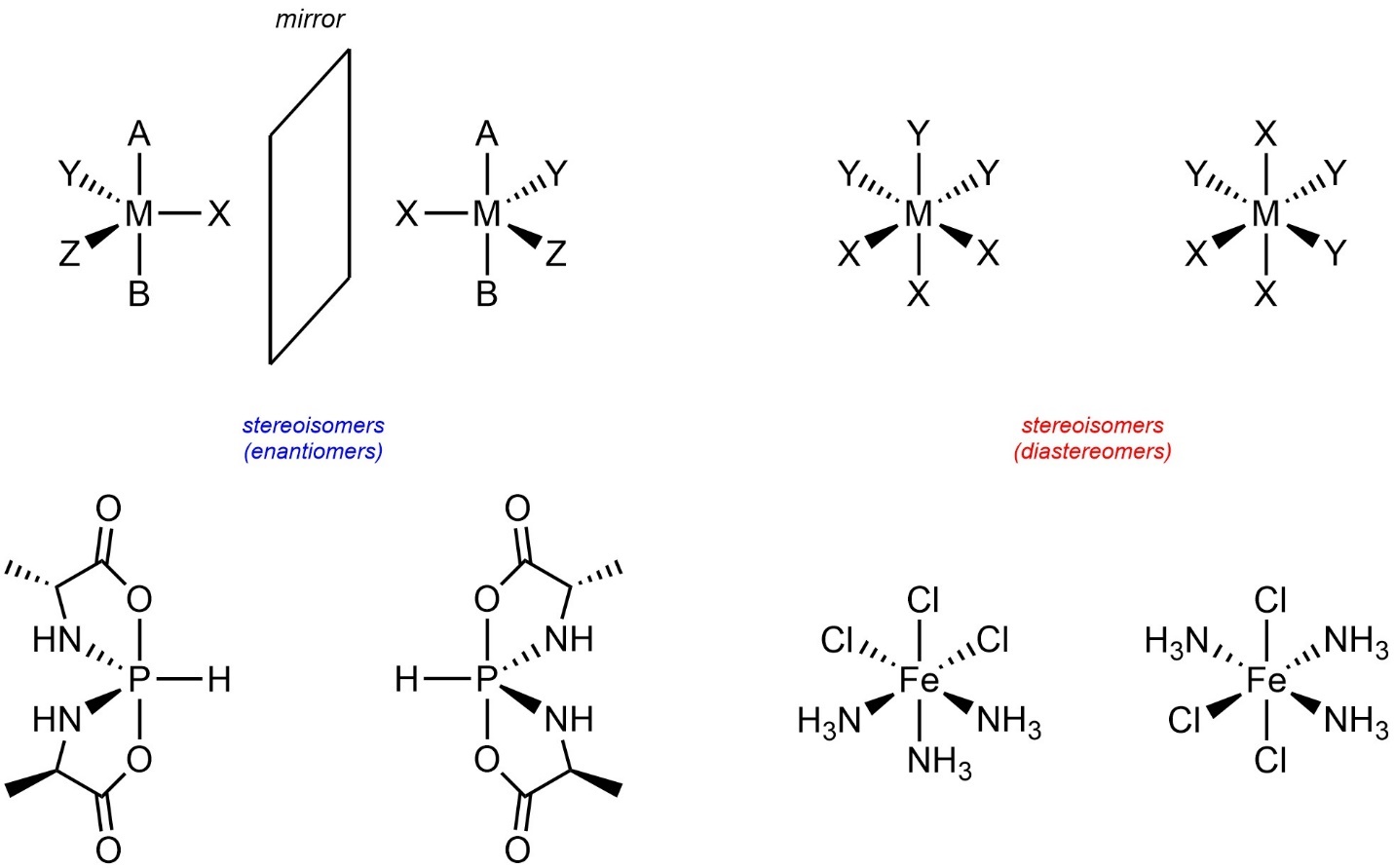
Figure 4.27 – Examples of Pentacoordinate and Hexacoordinate Stereocentres.
These are not classified using the R/S descriptors and have special rules for assigning their absolute configurations. As these are not common in organic chemistry these lie outside the scope of this text. However, it is important to understand that stereogenicity is possible in more advanced geometries and does not require “four different groups”.
4.3.3. Unusual Chirality by Hindered/Impossible Rotations
Stereogenicity and chirality are distinct concepts. As such, some molecules are chiral without any stereogenic centres. These typically have some feature that prohibits interconverting three-dimensional geometries by making some “normal” aspect of the molecule impossible.
Molecules may be chiral due to hindered/impossible rotations around σ bonds. These are sometimes referred to as atropisomers. For example, the compound BINOL exists as a pair of enantiomers (Figure 4.28). Full rotation around the central C-C bond is impossible because two hydrogens on the aromatic rings would have to physically pass through each other for rotation to occur.
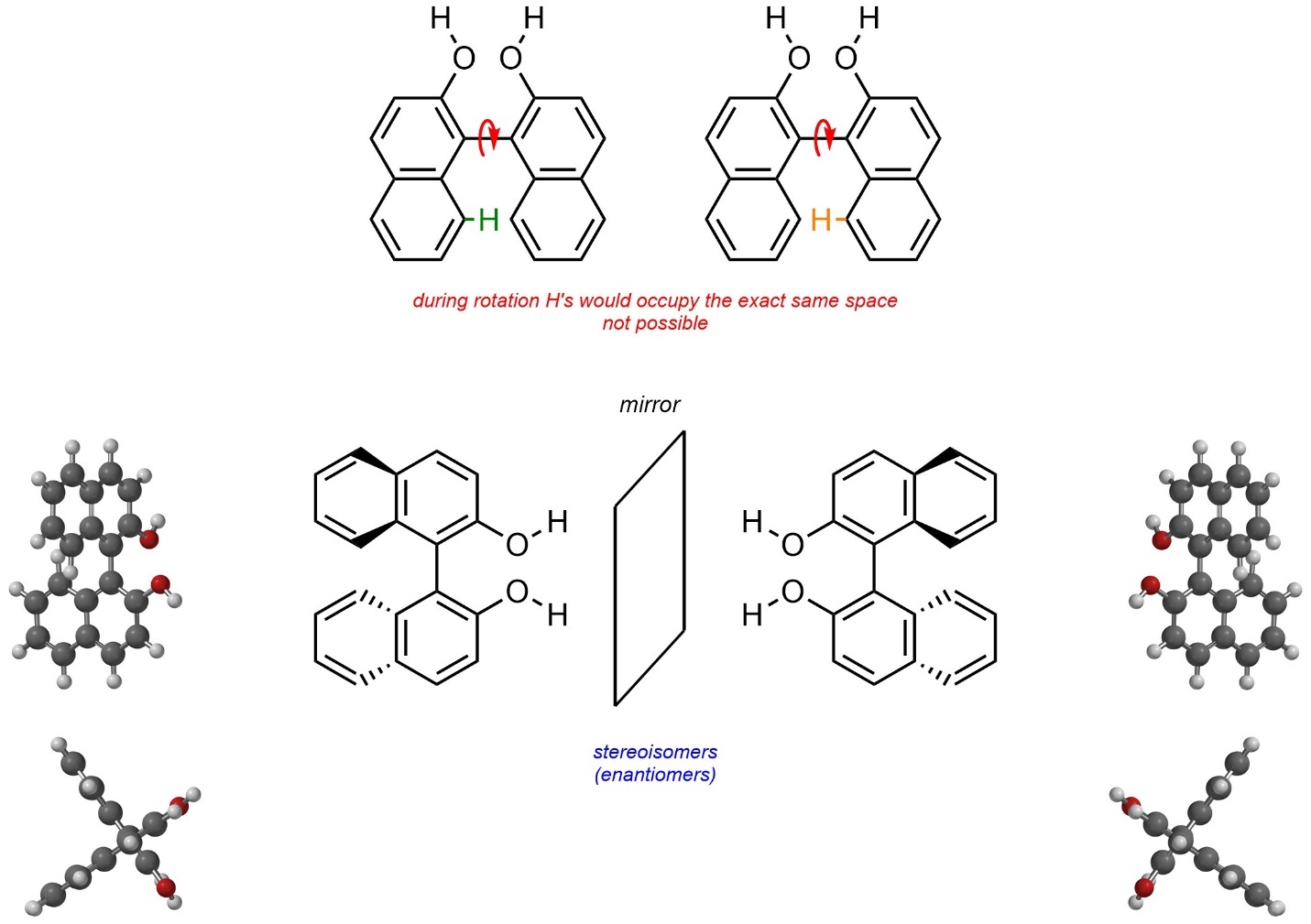
Figure 4.28 – Example of Chirality Through Hindered/Impossible Rotations.
Although interconverting the two stereoisomers of BINOL would require an impossible rotation, other examples only have (VERY) large energy barriers; the actual rotation is theoretically possible to achieve, just not realistic (hindered). Atropisomers may be classified as R or S, but use modified rules to do so. This lies outside the scope of this text.
4.3.4. Unusual Chirality by Hindered/Impossible Geometries
Molecules may be chiral due to hindered/impossible geometries. These are not atropisomers because there is no single σ bond about which rotation is hindered. For example, the compound [5]helicene exists as a pair of enantiomers (Figure 4.29). Planarity of the entire molecule is impossible because two hydrogens on the aromatic rings would have to physically occupy the same space. As a result, the ring spirals into a chiral helix shape.
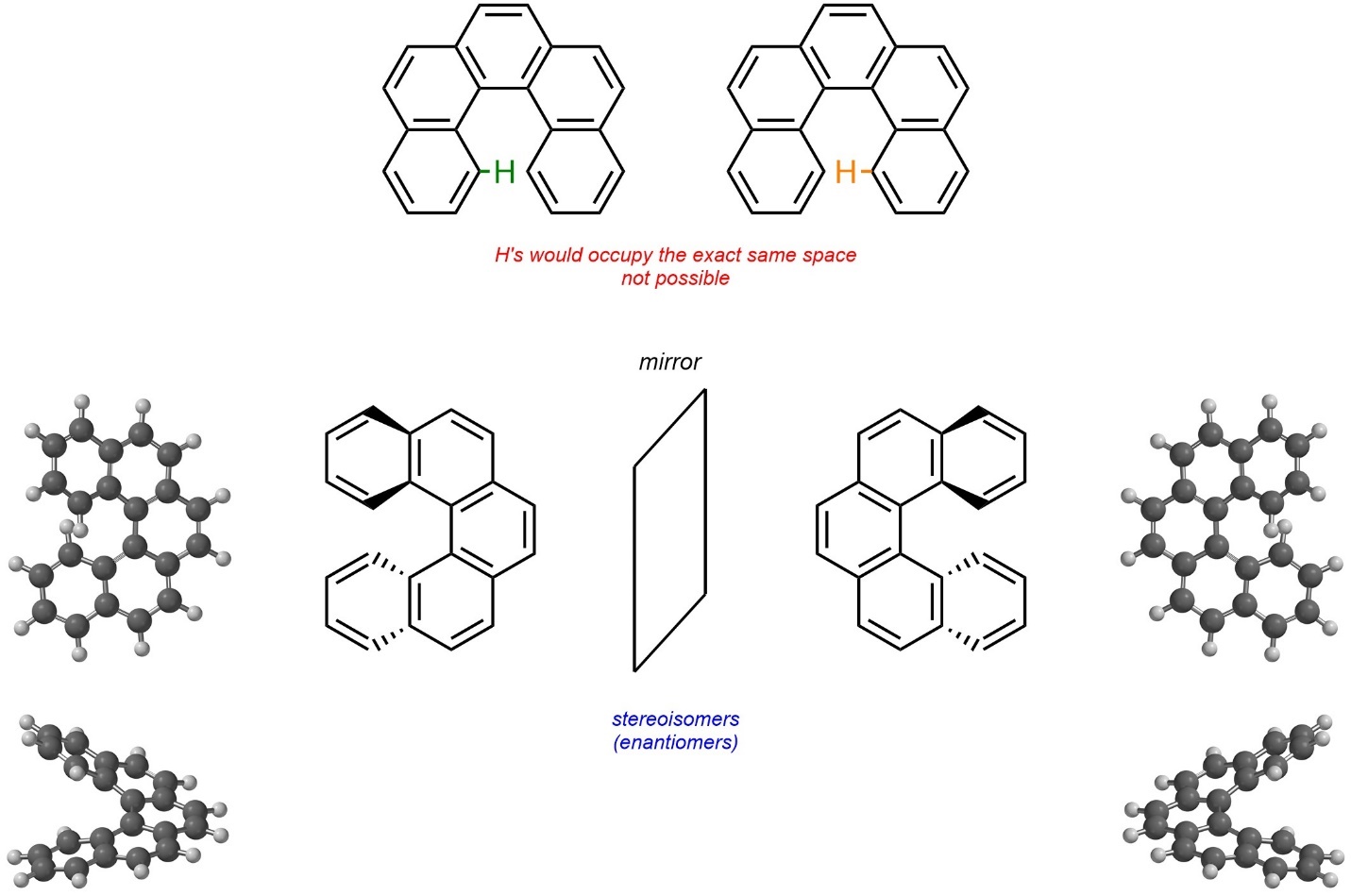
Figure 4.29 – Example of Chirality Through Hindered/Impossible Geometries.
Although interconverting the two stereoisomers of [5]helicene would require an impossible geometry, other examples only have (VERY) large energy barriers; the actual geometry is theoretically possible to achieve, just not realistic (hindered). Helicenes may be classified as M or P, but this nomenclature system lies outside the scope of this text.
Stereogenicity and chirality are distinct concepts. As such, some molecules are achiral despite having multiple stereogenic centres (Figure 4.30). Compounds that fit this description are referred to as being meso. It is important to recognize that meso is a description of a molecule, not a relationship between two stereoisomers.
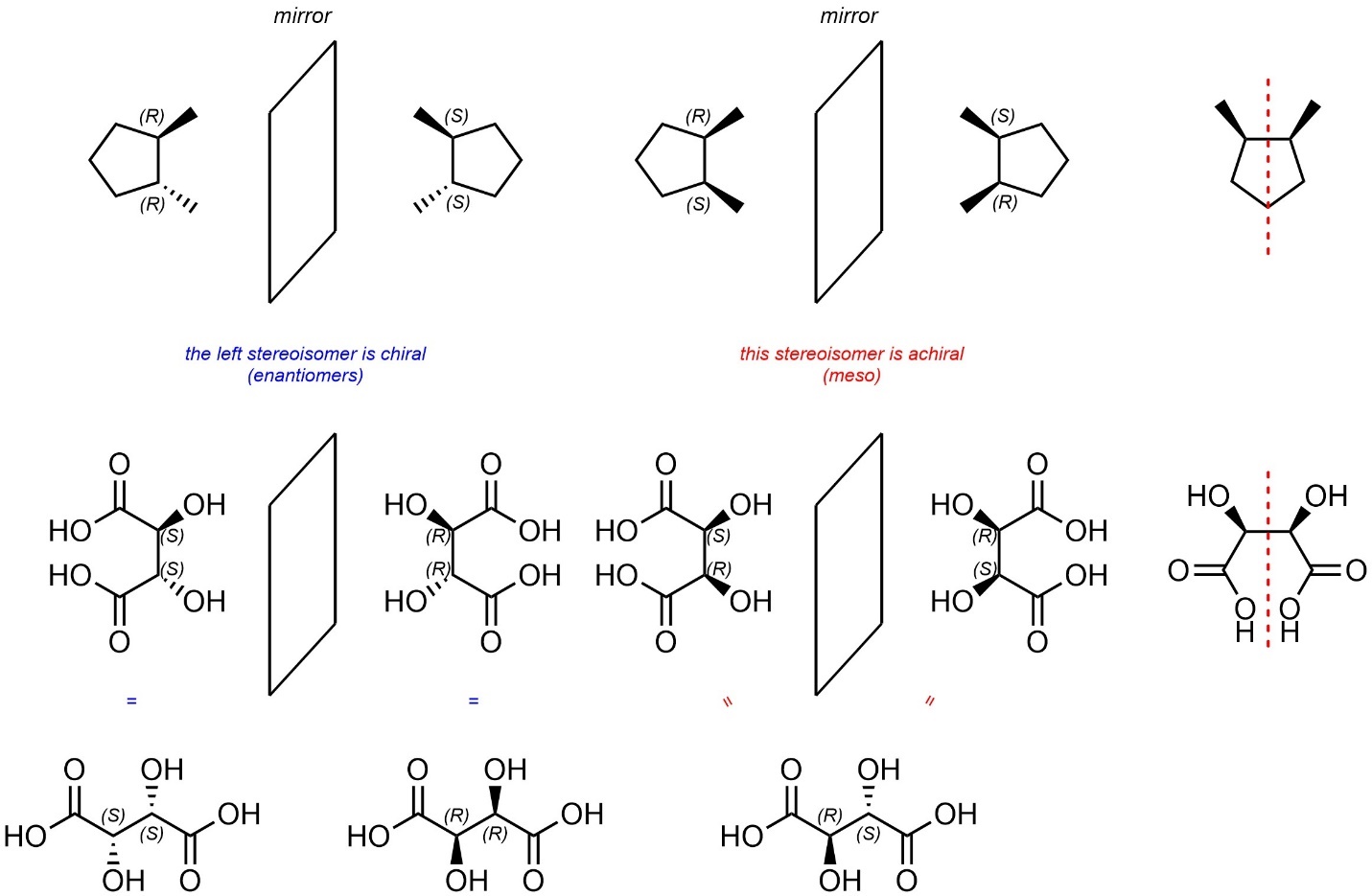
Figure 4.30 – Examples Contrasting Meso Compounds with Related Stereoisomers.
The easiest way to determine if a molecule is meso is to first identify if there is more than one stereocentre and then check if the molecule is achiral; if it has one or fewer stereocentres it cannot be meso.

